| Posted: Sep 02, 2016 |
Fast and furious bucket brigade - ultrafast proton transport in carbon nanotubes
(Nanowerk News) The movement of protons—hydrogen atoms with the electrons striped away—through a membrane is critical to efficiently producing electricity in a fuel cell. A decade ago, theory predicted that confinement of water into a single one-dimensional chain would achieve rapid proton transport, with the protons hopping down the chain of hydrogen-bonded water molecules.
|
|
Now tiny (less than a nanometer in diameter) carbon nanotubes that confine water into one-dimensional wires have validated this prediction of rapid proton transport and achieved rates an order of magnitude faster than protons in bulk water and faster than state-of-the-art fuel cell membranes (Nature Nanotechnology, "Ultrafast proton transport in sub-1-nm diameter carbon nanotube porins").
|
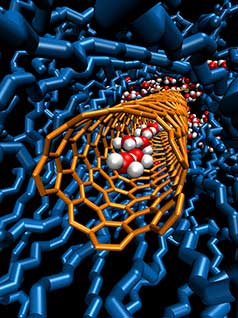 |
| Proton transport is faster in water if the water is confined in, for example, a sub-nanometer carbon nanotube straw (orange). The illustration shows one such straw embedded in a lipid membrane (blue). A very tight inner channel in the carbon nanotube squeezes water molecules (red (oxygen atoms) and gray (hydrogen atoms)) into a one-dimensional chain that allows for rapid proton transport along—an order of magnitude faster than in bulk water. (Image: Lawrence Livermore National Laboratory)
|
|
Carbon nanotube pores that create and maintain water “wires” could allow for rapid proton transport in next-generation bioinspired artificial proton conducting membranes for more efficient fuel cells to power your car and home.
|
|
Proton transport is important in biology and energy technology. Rapid proton transport is achieved through protons hopping along water molecules similar to a bucket brigade passing a pail of water down the line. Ten years ago, simulations predicted that water confinement in hydrophobic (“water-fearing”) carbon straws could achieve faster proton hopping, but demonstrating this efficient proton transport in artificial systems has been very challenging.
|
|
Scientists at Lawrence Livermore National Laboratory and the University of California-Merced have now prepared short carbon nanotubes with two different diameters embedded in synthetic lipid membranes that mimic biological ion channels.
|
|
When the diameter was greater than 1 nanometer, water was not confined to one dimension and, in this case, proton transport rates were comparable to bulk water. However, when much narrower (0.8-nanometer-diameter) tubes were used, the confined water formed one-dimensional water wires that drastically boosted the proton transport rate.
|
|
To prepare the narrow tubes, single-walled carbon nanotubes were sonicated to cut and purify them. The smaller diameter nanotubes readily incorporated into a lipid membrane and formed passageways across the membrane—roughly 17 nanotubes per 200-nanometer liposome (a spherical vesicle encapsulating water with a lipid bilayer). Changes in the intensity of a fluorescent dye trapped in the lipid vesicle membrane were used to monitor proton transport that was initiated by rapid acidification (increasing the proton concentration) of the solution surrounding the liposome.
|
|
The proton transport in the water “wires” inside narrow carbon nanotubes was an order of magnitude faster than bulk water and faster than the state-of-the-art membrane in fuel cells.
|

