| Posted: Dec 07, 2016 |
Novel label-free microscopy enables dynamic, high-resolution imaging of cell interactions
(Nanowerk News) Researchers from the University of Illinois at Urbana-Champaign have invented a novel live-cell imaging method that could someday help biologists better understand how stem cells transform into specialized cells and how diseases like cancer spread. The Photonic Crystal Enhanced Microscope (PCEM) is capable of monitoring and quantitatively measuring cell adhesion, a critical process involved cell migration, cell differentiation, cell division, and cell death.
|
|
"Our approach is important because there are not currently label-free and high-resolution imaging tools that allow cell-surface interactions to be quantified and imaged dynamically, although these processes are fundamental to things like wound healing, tissue development, tumor invasion, and cancer metastasis," said Brian Cunningham, a professor electrical and computer engineering and of bioengineering at Illinois.
|
|
Most conventional imaging methods rely on fluorescent dyes, which attach to and illuminate the cell components so they are visible under a microscope. However, fluorescent tagging has its limitations--namely that it is invasive, difficult for quantitative measurement, and only provides a short-term window of time for cell examination and measurement due to photo bleaching.
|
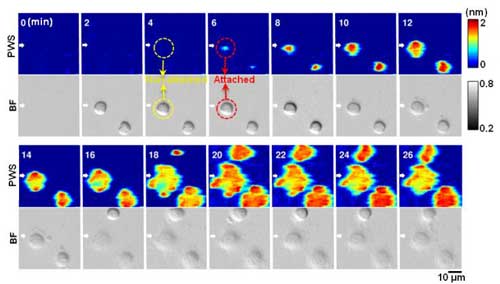 |
| Label-free and dynamic detection of stem cell adhesion uses the photonic crystal-enhanced microscope. (Image: Yue Zhuo, University of Illinois) (click on image to enlarge)
|
|
By using the PCEM, the researchers have successfully measured the effective mass density of cell membranes during stem cell differentiation, and cancer cell response to drugs in an extended period. Their results were reported in the journal Progress in Quantum Electronics ("Quantitative imaging of cell membrane-associated effective mass density using Photonic Crystal Enhanced Microscopy").
|
|
According to PCEM lead researcher Yue Zhuo, a post-doctoral Beckman Institute Fellow, fluorescent tagging doesn't allow scientists to see how a protein or cell changes over time.
|
|
"You can see the cell for maybe a few hours maximum before the fluorescent light dies out, but it takes several days to conduct a stem cell experiment," said Zhuo. "Scientists commonly use fluorescent tagging because there's no better way to monitor live cells due to their low imaging contrast among cellular organelles. That urges us to develop a label-free and high-resolution imaging method for live cell study."
|
|
The Illinois team's microscope functions with an LED light source and a photonic crystal biosensor made from inexpensive materials like titanium dioxide and plastic using a fabrication method like nanoreplica molding.
|
|
"Our sensor can be massively fabricated easily, and our cost to make the sensor is less than $1 each." noted Zhuo.
|
|
In Zhuo's apparatus, the photonic crystal biosensor is an optical sensor which can apply to any attachable cells. The sensor surface is coated with extracellular matrix materials to facilitate cellular interactions, which are then viewed through a normal objective lens and recorded with a CCD camera.
|
|
"The advantage of our PCEM system is you can see as the [live] cell is beginning to attach to our sensor, and we can quantitatively and dynamically measure what happened at that time," Zhuo said. "We're able to actually measure a very thin layer on the bottom of the cell that's about 100 nanometers, which is beyond the diffraction limit for visible light."
|
|
In the future, Zhuo plans to outfit the microscope with higher imaging resolution and someday hopes to be able to build a library of cell adhesion data for scientists.
|
|
"Different types of cells will have different dynamic attachment profiles." she explained. "We can use this library to screen different types of cells for tissue regeneration, disease diagnostic, or drug treatment, for example, see how diseased cells spread, or see how the cancer cells respond to different drug treatment."
|

