| Posted: Jan 05, 2017 |
The bond of two stars of chemistry: graphene and porphyrin
(Nanowerk News) Porphyrins, the same molecules that convey oxygen in haemoglobin and absorb light during photosynthesis, can be joined to the material of the future, graphene, to give it new properties. This was recently shown by a team of scientists at the Technical University of Munich, in which a Spanish researcher also participated (Nature Chemistry, "Fusing tetrapyrroles to graphene edges by surface-assisted covalent coupling"). The resulting hybrid structures could be used in the field of molecular electronics and in developing new sensors.
|
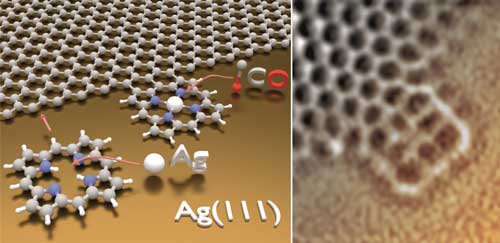 |
| When the porphyrin molecule heats up it loses its outer-layer hydrogen atoms and can bond to the graphene sheet on a surface of silver. The resulting structure (illustrated and viewed under the microscope in these images) endows the graphene with new properties. (Image: Yuanqin He - Technical University of Munich) (click on image to enlarge)
|
|
At the moment, it is difficult to find a material that attracts as much attention from scientists and engineers as graphene, which is made up of a layer of carbon atoms arrange in a hexagonal structure. It is flexible, extremely thin and clear, while being highly resistant and a conductor of electricity – ideal requirements for a number of uses, especially in the field of electronics.
|
|
However, using graphene to capture solar energy or as a gas sensor requires specific properties which it lacks, although it can acquire them by addition or functionalisation with certain molecules.
|
|
A team of researchers from the Technical University of Munich (TUM), led by Professor Wilhelm Auwärter, has succeeded in bonding an important biochemical group to the graphene sheet: porphyrins, protein rings which are part of chlorophyll, essential for photosynthesis in plants, and haemoglobin, which is responsible for conveying oxygen in animals’ blood.
|
|
As Spanish researcher Manuela Garnica Alonso, co-author of the study at the German university, tells SINC: “The new hybrid structures can be used in the field of molecular electronics – in which electronic circuits are composed of molecular units – as well as in catalytic processes in which numerous chemical reactions accelerate, and in the development of new gas sensors.”
|
|
The technique involves growing a graphene layer on a surface of silver to use its catalytic properties. Then, under ultra-high vacuum conditions, porphyrin molecules are added. These lose the hydrogen atoms from their periphery when heated on the metal surface, and they end up joining to the graphene edges.
|
|
“For the first time we have managed to covalently bond porphyrins to graphene edges, in other words to create stable chemical bonds, without altering their worthwhile properties,” Garnica explains.
The researchers used an atomic force microscope to characterise in detail the chemical structure of the molecules involved. With this tool they observed, for example, a metal being incorporated at the centre of the porphyrins, as well as the specific bond of gas molecules, such as carbon dioxide, without altering the graphene properties.
|
|
According to the authors, this new graphene ‘functionalisation’ technique could be extended to more molecules in the future, which would bond to various carbon nanostructures, like graphene nanoribbons, while also having great potential in the development of electronic applications.
|

