| Posted: Mar 30, 2017 |
Surface roughness accelerates liquid-liquid transition
(Nanowerk News) Researchers at the University of Tokyo have found that transition between two liquid states, characterized by different local structures, in a single-component substance can be accelerated by rubbing the cell’s surfaces, a widely used treatment method to align liquid crystal molecules in liquid crystal displays (Science Advances, "Impact of surface roughness on liquid-liquid transition").
|
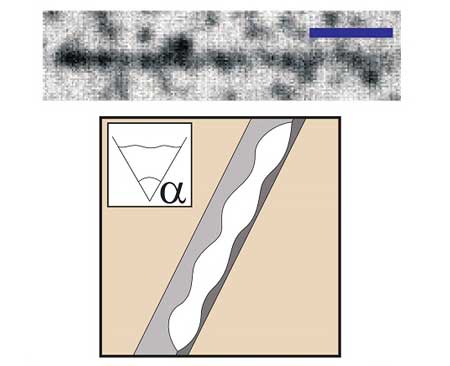 |
| Wedge formed by rubbing filled up with liquid 2. At top, a microscope image captures a wedge formed by rubbing that is completely filled with liquid 2 (bar corresponds to 10µm); at bottom is its schematic representation. (Image: Hajime Tanaka)
|
|
Altering the temperature induces liquid-liquid transition, based on the phenomenon that liquid 1 is more stable than liquid 2 at high temperature, while the reverse is true at low temperature, in which liquid 2 becomes more stable than liquid 1.
|
|
There are two types of kinetic processes for liquid 1 to transform into liquid 2: It involves either the nucleation-growth type of transformation, which occurs after a long incubation period following the temperature change; or the spinodal-decomposition type, which is initiated immediately after the temperature change, at low temperature.
|
|
Scientists have studied liquid-liquid transition mainly from the fundamental viewpoint until now, but its applications have remained unexplored.
|
|
The research group of Professor Hajime Tanaka and then-Project Researcher Ken-ichiro Murata (currently assistant professor at Hokkaido University) at the University of Tokyo’s Institute of Industrial Science found that confining an organic liquid, triphenyl phosphite, into a cell whose surfaces have been treated by rubbing leads to the disappearance of the incubation time even at a temperature where nucleation growth-type liquid-liquid transition is expected under usual conditions, thus accelerating its transformation significantly.
|
|
The group also revealed the mechanism: Nanometer-scale surface roughness formed by rubbing leads to the immediate formation of liquid 2 that is more friendly with the surface than liquid 1, even above the spinodal temperature. Furthermore, the researchers found that such rubbing treatments have no effect on spinodal-decomposition-type liquid-liquid transition.
|
|
The current finding serves as direct experimental evidence that there are two types of transformation and the transition between them takes place rather sharply at the spinodal temperature.
|
|
"Liquid-liquid transition has attracted considerable attention from the fundamental viewpoint because of its counterintuitive nature—that there exists more than two liquid states for a single-component substance and a transition occurs between them. However, there have been few studies on its applications," says Tanaka. He continues, "Our finding that surface treatments by rubbing significantly accelerates liquid-liquid transition may open a new avenue towards applications of liquid-liquid transition phenomena."
|

