| Posted: Apr 14, 2017 |
New energies in gas sensing
(Nanowerk News) Gas detectors capable of sensing minute quantities of pollutants could help better monitor air quality. KAUST researchers have discovered a two-dimensional electronic material that exhibits high sensitivity to gas molecules, such as carbon dioxide (CO2), nitrogen oxides (NOx) and ammonia (NH3).
|
|
Atomically thin sheets consisting of transition metals associated with chalcogen atoms, such as sulfur, selenium and tellurium, are versatile alternatives to the more conventional silicon-based semiconductors. Depending on their metal component, these transition metal dichalcogenide monolayers have band gaps—energy barriers that limit electron flow through a material—that can be tuned to alter their electronic properties.
|
|
The unique electronic properties of these monolayers have potential to improve a plethora of devices, including field-effect transistors, photodetectors and gas sensors.
|
|
Semiconducting monolayers are proven to be ideal candidates as gas sensing materials because they have a high surface-to-volume ratio. For example, MoS2 has been incorporated in field-effect transistors to detect nitrogen monoxide. However, its performance is limited by its relatively low carrier mobility or by the velocity at which its electrons (or holes) move when subjected to an electric field.
|
|
To overcome these shortcomings, KAUST Professor Udo Schwingenschlögl’s team evaluated the potential of the platinum dichalcogenide PtSe2 for use in gas detectors via sophisticated computational techniques (Advanced Materials Interfaces, "Superior gas sensing properties of monolayer PtSe2").
|
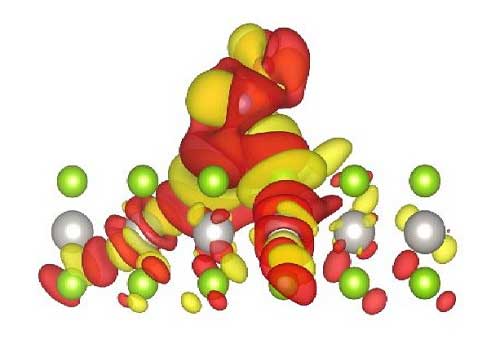 |
| Atomistic model showing the charge accumulation (yellow) and depletion (red) upon NO adsorption on PtSe2 monolayer. Platinum atoms appear in gray and selenium atoms are shown in green. (© Wiley)
|
|
“Monolayer PtSe2 experimentally shows a high carrier mobility, which can be advantageous for gas sensing,” said Schwingenschlögl, adding that this material had not previously been considered for this purpose. This approach shows the interaction between monolayer and gas molecules at both structural and electronic levels.
|
|
First, the researchers built a model monolayer composed of selenium atoms that formed octahedral arrangements with one platinum atom at their center. Next, they determined the optimal geometry adopted by individual gas molecules, such as NOx, NH3, H2O, CO2 and CO, upon adsorption. They assessed the capacity of these adsorbed molecules to transfer charge to the monolayer by examining adsorption-induced changes in the electronic properties.
|
|
These calculations provided high adsorption energies, indicating strong affinity between monolayer and gas molecules. All adsorbed molecules altered the monolayer charge (see image), which is key for the gas-sensing ability of monolayer PtSe2.
|
|
Furthermore, their interactions were more effective with monolayer PtSe2 than its MoS2 or carbon-based graphene analogues. “It was exciting to explain this difference at a molecular orbital level,” said Schwingenschlögl. Calculations of electron transport revealed the high sensitivity of monolayer PtSe2 as a gas sensor.
|
|
Schwingenschlögl explained, “We are currently looking for experimental collaborators to implement our concept.”
|

