| Posted: May 03, 2017 |
Super P carbon black for reversible lithium and sodium ion storage
(Nanowerk News) Nowadays lithium ion batteries play a dominant role in the rechargeable battery market. Sodium ion batteries have recently attracted increasing interest as an alternative to lithium ion batteries due to the high natural abundance of sodium compared to lithium.
|
|
Super P carbon black (SPCB), produced from partial oxidation of petrochemical precursors exhibiting a large specific surface area and superb electrical conductivity, is the most commonly used conducting additive in the lithium/sodium ion battery electrodes to improve the electronic conductivity. However, very limited knowledge on the structure, electrochemical properties and reaction mechanisms for lithium/sodium ion uptake has been reported till now.
|
|
In an article coauthored by researchers at Delft University of Technology, Renmin University of China and the National Center for Nanoscience and Technology of China, a comprehensive study on the structure and electrochemical properties of SPCB has been reported. The article titled "The electrochemical performance of super p carbon black in reversible Li/Na ion uptake" has been recently published in the journal Science China Physics, Mechanics & Astronomy ("The electrochemical performance of super P carbon black in reversible Li/Na ion uptake").
|
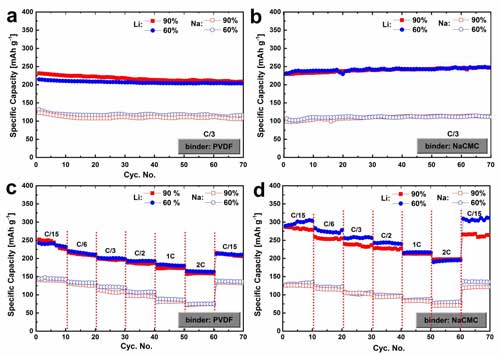 |
| This image shows electrochemical lithium/sodium ion storage performance of the SPCB based electrodes containing 90 percent and 60 percent of active materials (C = 300 mA g-1). (©Science China Press) (click on image to enlarge)
|
|
To uncover the structure and morphology of SPCB, these researchers utilized scanning electron microscopy (SEM), Raman spectroscopy and X-ray diffraction (XRD). The SPCB appears as porous, flocculent, cross-linked carbon nanothreads; it is largely amorphous and contains substantial structural disorder, i.e. low graphitization level.
|
|
The electrochemical performance of SPCB (Figure 1) shows that SPCB exhibits a considerable capacity for both lithium and sodium ion storages, a high cycling stability and excellent rate capability. It is demonstrated that SPCB achieves a higher capacity for lithium ion than sodium ion storage.
|
|
A reversible capacity (up to 310 mAh g-1) was reported for lithium ion uptake, while 145 mAh g-1 was achieved for sodium ion storage. The authors ascribe it to the different reaction mechanisms between lithiation and sodiation of SPCB.
|
|
The lithium ions are intercalated in the graphite structure of SPCB forming graphite intercalation compounds (GICs), which is, however, not favorable for sodium ion insertion. This article indicates that, due to the low graphitization level of SPCB, sodium ion uptake in SPCB may follow the "card-house" model in which two stages are present: sodium ion intercalation in the layers between the graphene sheets and sodium plating in the pores between the nano-graphitic domains.
|
|
The work has also, for the first time, investigated the influence of binder type and content on the electrochemical lithiation and sodiation performance of SPCB. The water-soluble NaCMC binder based electrodes shows a superior cycling stability compared to the electrodes using the mainstream binder in battery research and commercial products, PVDF.
|
|
"This can be probably attributed to not only the superior adhesive quality and elasticity of NaCMC but the formation of a more compact SEI layer upon the presence of FEC in the electrolyte", they explained, which helps to maintain the structural integrity during cycling and thus to achieve a higher cycling stability. |
|
Meanwhile, this article demonstrates that the Coulombic efficiency increases with higher binder content in the electrode; increasing binder amount also leads to the better retained structural integrity during cycling.
|
|
The lithium ion insertion and extraction in SPCB mainly occurs at a low voltage range showing a sloping voltage < 1.2 V; while the de-/sodiation takes place at an even lower voltage range < 0.8 V. The sodiation voltage profile has a relatively higher voltage slope and a voltage plateau close to 0 vs. Na/Na+, which is consistent with the Na ion insertion following the "card-house" model.
|
|
"The consequence of the sloping voltage profiles", wrote the researchers, "will be that in a low potential lithium/sodium ion anode (e.g. Li-Si or Na-P using SPCB as a conducting additive) the SPCB will largely be lithiated or sodiated at a higher potential than where the main lithiation or sodiation of the active materials takes place. For higher potential anodes (such as Li4Ti5O12) or cathodes no lithiation or sodiation of SPCB will take place."
|
|
Providing the common utilization of SPCB in battery research, the researchers stated, "The electrochemical lithium/sodium ion uptake properties of SPCB reported in this article will provide an essential reference for the research on lithium and sodium ion battery electrodes that utilize significant amounts of SPCB as a conductive additive".
|

