| Posted: May 12, 2017 |
Atom-scale 'Lego' could keep scientists busy 'for next 50 years'
(Nanowerk News) Atom-scale building blocks that have been compared to microscopic Lego are allowing researchers to play with the properties of common materials, and the possibilities are so great that it could keep scientists busy for the next 50 years.
|
|
From the Stone Age to Silicon Valley, materials have defined the technological capabilities of civilisations.
|
|
Professor Andre Geim at the University of Manchester in the UK is well acquainted with the toolbox available today. In 2010, he was awarded the Nobel Prize in Physics for extending it with an exotic form of carbon known as graphene.
|
|
Unlike materials sourced from nature, graphene is a creation of science. It is peeled off graphite in honeycomb motifs as thin as a single atom. The quantum laws prevailing at these tiny scales cause electrons to move through graphene in unusual ways.
|
|
‘Graphene can be stronger than steel, more conductive than copper and as transparent as glass,’ said Prof. Geim. ‘It is unlike any substance found in nature.’
|
|
Now, as part of the ARTIMATTER project funded by the EU’s European Research Council, Prof. Geim is tailoring matter with even more outlandish features by stacking graphene on top of other atomically thin materials.
|
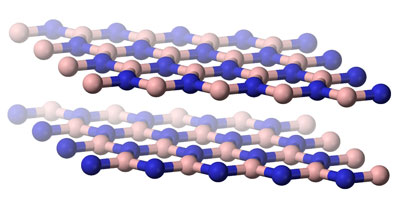 |
| Atomically thin materials can be stacked on top of each other to create matter with remarkable physical properties. (Image: public domain)
|
|
Mixing and matching two-dimensional layers made from different elements gives rise to remarkable physical properties. According to Prof. Geim, the right combination of building blocks can turn insulating materials into conductors, tune the colours that they absorb, and synchronise the behaviour of electrons inside them.
|
|
These capabilities stem from deep alterations in how the materials behave. Harnessed properly, they might overcome established barriers in modern electronics, such as reducing the response time of far-infrared detectors, or maybe even sustaining superconductivity at room temperature.
|
|
The novel building blocks also provide tools to test scientific theories and explore new phenomena. What we learn from their eccentricities could impact future technology as profoundly as semiconductor physics has transformed the computing and telecommunications sector today.
|
Infinite possibilities
|
|
‘Scientifically speaking, graphene is done. We now understand how it works and are finding applications for it,’ said Prof. Geim. ‘But the possibilities of combining graphene with other atomically thin materials are nearly infinite. I don’t see this Lego work being finished anytime in the next 50 years.’
|
|
One reason it is hard to anticipate the potential outcomes of nanoscopic building blocks is that the computers calculating how they fit together are not powerful enough to take their full complexity into account.
|
|
Dr Barbara Capone of the University of Vienna, Austria, and Roma Tre University, Italy, is working on polymers – long chains of atoms that repeat millions of times the same sequence.
|
|
Although data processors can predict how these building blocks behave when they are alone or in dense bunches, they cannot follow reactions that take place when sparse polymer concentrations intermix.
|
|
‘We can simulate how individual atoms behave in single molecules, and for dense concentrations, we can average out billions of kinks and quirks,’ said Dr Capone. ‘But what happens between these extremes remains mysterious because there are too many molecules to track and too few to generalise from.’
|
Bite-sized
|
|
Dr Capone has spent years refining statistical methods in theoretical physics to help computers come to terms with the complexity. Rather than follow each piece of the puzzle simultaneously, she groups reactions into bite-sized regions and models interactions between their local averages. When applied to sparse polymer concentrations, her simplifications are revealing gems among the disorder.
|
|
‘These polymers are remarkable building blocks,’ said Dr Capone. ‘Depending on how long and dense we make them, or how we graft the chains to each other, they fold into completely different shapes.’
|
|
In principle, the right mix of ingredients could spontaneously form into the cubic columns of common semiconducting crystals, the amorphous network of glass, or even the honeycomb structure of graphene.
|
|
This is welcome news for anyone working on materials for electronics. Sculpting the perfect atomic grids necessary to build high-quality transistors or solar cells currently requires vast amounts of time and energy.
|
|
Colleagues of Dr Capone are taking experimental steps towards another application. As part of the EU-funded NANODRIVE project, they will produce star-shaped polymers that collapse upon reacting with a given compound and then release their cargo upon reaching a desired environment.
|
|
This is essentially how drugs deliver chemicals, only they do so with tortuously complicated molecules. Simplifying the components could make the process more economical and versatile.
|
|
‘The opportunities are endless,’ said Dr Capone, who will be launching NANODRIVE this month. ‘With a few adjustments, these polymers could form structures that encapsulate pollutants and filter them out of drinking water.’
|
|
Dr Capone says she is excited both about the social benefits that such a technology could bring to emergency situations and the insight that basic building blocks could offer on physical, chemical and biological processes taking place at the nanoscale.
|
|
‘I have always been interested in finding the simplest way of doing something complicated,’ said Dr Capone. ‘It is often the best way to understand how it works.’
|

