| Posted: May 17, 2017 |
Better cathode materials for lithium-sulphur-batteries
(Nanowerk News) At present, lithium batteries are one of the best solutions for storing electrical power in a small space. Lithium ions in these batteries migrate from the anode to the opposite electrical pole, the cathode, during the discharge cycle. The anode and cathode generally consist of heavy-metal compounds that are expensive and toxic.
|
|
One interesting alternative is the lithium-sulphur battery. In this case, the cathode does not consist of heavy metals, but instead of sulphur -- an economical and widely available material. As lithium ions migrate to the cathode during the discharge cycle, a reaction takes place there that forms lithium sulphide (Li2S) via various intermediate lithium polysulfides. During cycling, dissolution of lithium polysulfides causes the battery's capacity to decline over the course of multiple charging cycles via the so-called "shuttle effect".
|
|
For this reason, researchers the world over are working to improve cathode materials that would be able to chemically or physically confine or encapsulate polysulphides, such as with nanoparticles made of titanium dioxide (TiO2), for example.
|
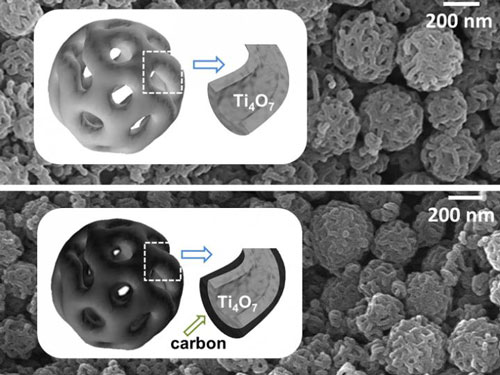 |
| The porous structure of the nanoparticles is visible under the electron microscope. (Image: HZB)
|
Ti4O7-nanoparticles with interconnected pore structure
|
|
The HZB team headed by Prof. Yan Lu has now fabricated a cathode material that is even more effective. Here as well, nanoparticles provide confinement of the sulphur (Advanced Functional Materials, "Porous Ti4O7 Particles with Interconnected-Pore Structure as a High-Efficiency Polysulfide Mediator for Lithium–Sulfur Batteries").
|
|
However, they do not consist of titanium dioxide, but instead of Ti4O7 molecules arranged on a porous spherical surface. These porous nanoparticles bind polysulphides substantially more strongly than the usual TiO2 nanoparticles.
|
|
"We have developed a special fabrication process to generate this complex, three-dimensionally interconnected pore structure", explains Yan Lu. Yan Lu first fabricates a template made of a matrix of tiny polymer spheres that have porous surfaces. This template is prepared in additional steps, then submerged in a solution of titanium isopropoxide. A layer of Ti4O7 is formed on the porous spheres and remains after thermal treatment, which decomposes the underlying polymer. Compared with other cathode materials made of titanium oxides, the Ti4O7 nanosphere matrix possesses an extremely large surface area. 12 grams of this material would cover a football field.
|
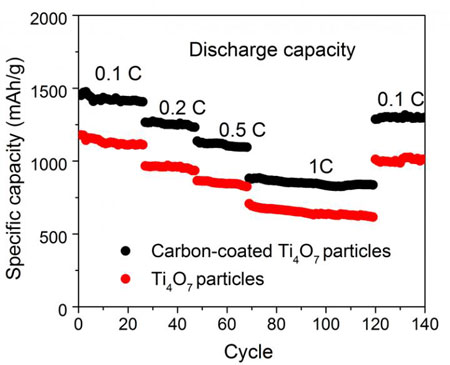 |
| The specific capacity declines very little during repeated charge/discharge cycles. (Image: HZB)
|
Function decoded at BESSY II
|
|
X-ray spectroscopy measurements (XPS) at the CISSY experiment of BESSY II show that sulphur compounds bind strongly to the surface in the nanomatrix.
|
High specific capacity
|
|
This also accounts for the high specific capacity per gramme (1219 mAh) at 0.1 C (1 C = 1675 mA g-1). The specific capacity also declines very little during repeated charge/discharge cycles (0.094 per cent per cycle). By comparison, the specific capacity of cathode materials made of TiO2 nanoparticles is 683 mAh/g. To increase the conductivity of this material, it is possible to apply a supplementary coating of carbon to the nanoparticles. The highly porous structure remains intact after this process.
|
Upscaling is feasible
|
|
"We have been working to improve the repeatability of this synthesis for over a year. Now we know how to do it. Next, we will work on fabricating the material as a thin-film", says Yan Lu. And the best part: in this case, what has been successful in the laboratory can also be transferred to commercial manufacturing. This is because all the processes, from the colloid chemistry to the thin-film technology, are scalable.
|


