| Posted: May 17, 2017 |
Self-assembly forces in crystals
(Nanowerk News) Look closely enough, and you'll see ingenious patterns everywhere in nature. Scientists and engineers have long understood this, but mimicking Mother Nature in building such patterns—especially highly ordered crystal structure—has proven challenging.
|
|
Recently, Maria Sushko and Kevin Rosso at Pacific Northwest National Laboratory (PNNL) significantly advanced understanding by clarifying the driving forces behind particle-based crystal growth with their new computational approach (Nanoscale, "The origin of facet selectivity and alignment in anatase TiO2 nanoparticles in electrolyte solutions: implications for oriented attachment in metal oxides").
|
|
They learned that crystal growth depends on the subtle balance of interactions between atoms, ions, molecules, and particles. Their discovery holds significant promise for creating materials to address energy challenges.
|
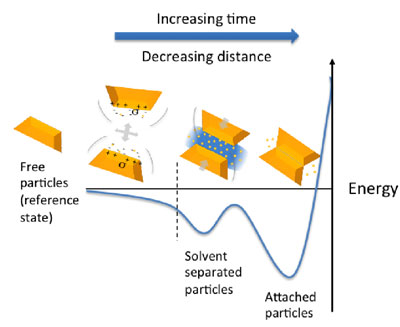 |
| As particles move in solution, they start “feeling” interactions from distances comparable with their size. Correlated motion of ions drives nanoparticles to approach each other facing matching sides. Then the same ion-induced forces direct fine adjustments of the particles’ mutual orientation so that atoms on the surfaces are arranged as an ideal crystal. Then solvent leaves the gap between the particles, and a defect-free crystal is formed.
|
|
In natural crystal-growth processes, nanoparticle building blocks attach along specific crystal faces. Studying these examples, researchers were inspired to contemplate how they might create similar crystal structures for a range of practical applications including energy storage.
|
|
Armed with a greater understanding of the fundamental processes underlying the pathways of crystal growth, researchers could control these processes to synthesize new materials with precise detail. In their research, Sushko and Rosso found that coordinated motion of ions close to nanoparticle surfaces drive the way nanoparticles arrange into matching crystal shapes and structures.
|
|
They discovered that ions in solution can direct the rotation of nanoparticles into a matching crystal orientation-mimicking nature's pattern precisely-to produce perfect crystals.
|
Why It Matters
|
|
The PNNL researchers' discovery provides key fundamental insights into geochemical processes leading to mineral formation, and helps to create complex, hierarchical, single-crystal structures in the lab. It also holds promise for eventually creating innovative materials for consumer electronics, batteries, and more. According to Sushko, their new computational approach creates "a new paradigm in knowledge-based synthesis of highly ordered three-dimensional crystal structures" for a range of practical applications in catalysis and energy-storage technologies.
|
Methods
|
|
Rosso and Sushko developed a new multi-scale computational model that encompasses the essential forces acting between atoms, molecules, and particles. Their approach spans the length scales from Angstrom to half a micron and is fully transferable to a wide range of systems. The method is rooted deeply in quantum mechanics and provides a parameter-free approach for modeling experimentally relevant systems.
|
What's Next?
|
|
Their new computational approach is a major step towards developing a comprehensive theory of particle-based crystallization. Future research will extend the model to include a broader range of macroscopic forces, such as magnetic and electric polarization. The model will be also further applied to other materials to get insight into various crystallization pathways.
|

