| Posted: Jun 29, 2017 |
Developing tailor-made nanoparticles to fight cancer
(Nanowerk News) Electronic devices, coatings or biomedical therapeutics – nanoparticles, smaller than a human hair, can have very different properties and thus broad application options. The respective function depends primarily on the size of the particles.
|
|
An interdisciplinary research group, including members of the priority research area "Kiel Nano, Surface and Interface Science" and the Cluster of Excellence "Inflammation at Interfaces" at Kiel University (CAU), has developed a method to produce size-tailored particles of zinc peroxide. This allows targeted modification of their properties, such as the destruction of cancer cells.
|
|
The initial results, recently published in the journal Nature Communications ("Underwater Leidenfrost nanochemistry for creation of size-tailored zinc peroxide cancer nanotherapeutics"), indicate a potential use as a nanotechnology to fight cancer in the long term.
|
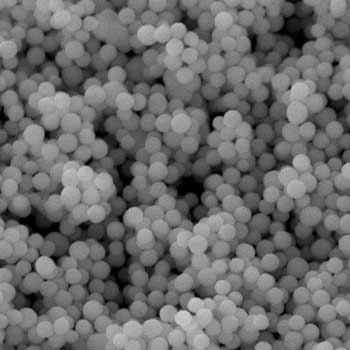 |
| Under the scanning electron microscopy it can be observed that: the resulting particles are spheres made of zinc peroxide. (Image: Mady Elbahri)
|
|
For their new method of fabricating nanoparticles the researchers made use of the so-called Leidenfrost effect: if a drop of water falls on a surface that is very much hotter than the boiling point of water, it begins to “hover” or levitate above the surface, until it is fully vaporised. The water droplet can be used as a micro-reactor, in which small nanoparticles form themselves.
|
|
Materials scientist and chemist, Mady Elbahri, has been working on “Leidenfrost chemistry” for years, which is named after this principle. "From my previous research work in materials science, I knew that the size of the particles is decisive for their properties," said Elbahri. "I wanted to transfer this idea to the synthesis of zinc peroxide spheres at a nano-scale." As a member of the Collaborative Research Centre 677 "Function by Switching", Elbahri has researched at Kiel University since 2009 as Professor of Nanochemistry and Nanoengineering, and as of late also at Aalto University in Helsinki.
|
|
Zinc peroxide is regarded as a particularly effective oxygen supplier, even more than simple zinc oxide. This makes it an interesting material for treating diseases such as cancer. For zinc oxide, there are already studies showing that it increases the proportion of reactive oxygen in cells excessively, and that this "oxygen supersaturation" leads to cell death.
|
|
In order to specifically produce zinc peroxide particles of a certain size, the researchers looked closely at how the Leidenfrost effect works under water, in particular, such as with deep-sea volcanic activity. They transferred these principles to their work in the laboratory. For synthesising the particles, they used a water bath on a stove, rather than individual water droplets. At the bottom, they introduced a zinc acetate aqueous solution mixed with hydrogen peroxide. Here, it is particularly hot and there is a high concentration of ions - ideal conditions for the formation of particles.
|
|
The resulting particles thereafter rose to the colder area of the water bath, where they formed clusters and all grew to the same size.
|
|
Only by separating the nucleation and growth steps it is possible to create equal-sized particles in large numbers in a controlled manner. By changing the composition of the zinc acetate solution, the researchers varied the particle size in seven stages, from 70 to 680 nanometres.
|
|
"In our experiments, we were able to demonstrate that nanoparticles of zinc peroxide damage cancer cells, and that this effect depends strongly on their size - in addition to the types of cancer cells and the concentration of the nanoparticles,” summarised Elbahri. Their new method of fabricating nanoparticles has another advantage: In contrast to the conventional processes no chemical additives are required as it only takes place in water, continued Elbahri. "It thereby fulfils an important prerequisite for therapeutic use."
|
|
To ensure that the small particles are really spherical zinc peroxide, the samples were examined under a transmission electron microscope at the CAU’s Faculty of Engineering. However, the electrons used in this method decompose the material with the release of oxygen. Nevertheless, by means of high-speed electron diffraction, it was possible to prove that the substance is zinc peroxide.
|
|
Lorenz Kienle, Professor of Synthesis and Real Structure, explained an interesting secondary aspect of the investigation: “Through the release of the oxygen, the spherical zinc peroxide particles decompose into smaller zinc oxide particles, which arrange themselves into hollow spheres. We observed this spontaneous change in their form time-resolved for the first time. This enabled us to correct earlier findings on the formation mechanism of hollow zinc oxide spheres.”
|
|
The biological tests were carried out in the laboratory of Professor Dieter Adam of the Kiel Collaborative Research Centre 877 “Proteolysis as a Regulatory Event in Pathophysiology”, and a member of the Cluster of Excellence "Inflammation at Interfaces". Adam is part of the Kiel Oncology Network at the CAU, which amongst other things researches the development of tumours.
|
|
"The first experiments with the new synthesis method have shown that zinc peroxide is a very exciting material for potential medical applications. This is a very promising start for further studies, which could help to improve cancer treatments," he interprets the results. The next research steps are to explore the exact mechanisms behind the cell damage for various tumour types.
|

