| Posted: Aug 08, 2017 |
Making an ultra-small silicon 'chip'
(Nanowerk News) One of the challenges in making new electronic devices is the chemistry required to assemble the layers that make up the device.
|
|
In this research (Angewandte Chemie International Edition, "Synthesis of a fragment of crystalline silicon: Poly(cyclosilane)"), a new synthetic chemistry approach produces ultra-small materials that resemble a fragment of the semiconductor silicon.
|
|
The process uses a precisely defined pattern of reactive sites, or chemical “hooks.” The hooks control the structure as the material, a polymer, grows. Being able to create small pieces of silicon-like materials may make it easier to manufacture designer electronic circuits with properties tuned for specific uses.
|
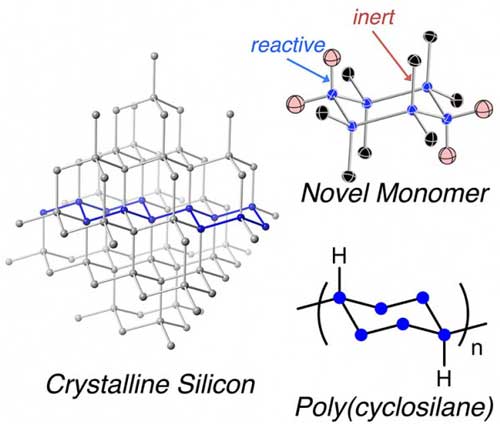 |
| Finding new ways to make better materials for electronic circuits is one way to make a better device. A new polymer, called poly(cyclosilane) (bottom right), is essentially an ultra-small silicon “chip” that resembles a fragment of the atomic lattice of silicon (left), a foundational material found in most electronics. The chemical synthesis of the new polymer was possible due to a specially designed, novel building block (monomer) (top) that has a pattern of chemical connection points that allow growth of the polymeric structure in only the desired direction. (Image: Dr. Rebekka Klausen, Johns Hopkins University)
|
|
Silicon is a semiconductor. It is essential to defining modern technologies such as computer chips and solar cells. Access to new forms of this semiconductor can lead to new material properties. This research provides a synthetic chemical route to new forms of silicon-like materials. The resulting materials could change solar cells and computers.
|
|
Rational synthesis is the strategic choice of a sequence of chemical reactions leading to a desired chemical target. The application of rational synthesis leads to the creation of innovative and well-defined materials inaccessible by traditional manufacturing methods, such as cutting, machining and polishing.
|
|
However, the small number of synthetic methods available to chemists to make silicon has limited the role of rational synthesis in developing new forms of this essential semiconductor that is found in virtually all modern electronics.
|
|
Researchers at the Johns Hopkins University addressed this challenge by developing new approaches to making molecules having cyclic silicon structures, as well as new methods for converting them to bulk material forms such as thin films or fibers.
|
|
Chemists at Johns Hopkins University synthesized a new class of polymers they named poly(cyclosilane). The new class of polymers contain repeating building blocks (monomers) that resemble part of the internal structure of elemental silicon. The cyclosilane monomer contains a precisely defined pattern of reactive sites, or chemical “hooks,” that force the building block monomers to assemble in only one way.
|
|
Poly(cyclosilane) exhibits promising optical properties and can be integrated with other electronically active materials to prepare novel functional materials. Such materials may have future utility in applications such as solution-processable solar cells or other electronic devices.
|

