| Posted: Sep 25, 2017 |
Atomic-scale movies reveal that catalyst nanoparticles undergo internal changes to forge carbon nanotubes (w/video)
(Nanowerk News) Lights, camera, catalysis!
|
|
Researchers at the National Institute of Standards and Technology (NIST) have made some of the first movies of the structural changes in tiny catalyst particles that may someday help them more efficiently build miniature electronic circuits and other nanotech devices. The movies may suggest new ways to custom-design the catalyst nanoparticles, leading to more efficient production and improved control of the materials they help fabricate.
|
|
Catalysts are critical for many chemical reactions because they provide speedy, low-energy pathways to break and make chemical bonds necessary for the reactions to proceed. Catalyst nanoparticles are a mainstay for such industrial processes as converting crude oil to gasoline and growing single-walled carbon nanotubes—super-strong arrangements of carbon atoms.
|
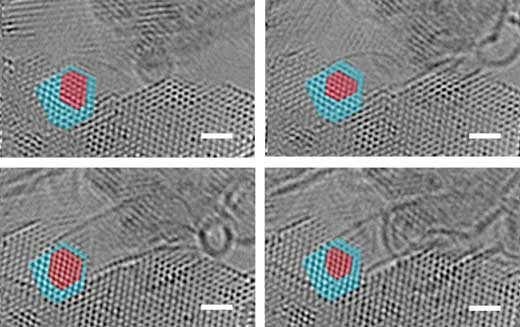 |
| Evidence of atomic-scale structural fluctuations in a cobalt nanoparticle that acted as a catalyst for the growth of single-walled carbon nanotubes. Micrographs are high-resolution snapshots of the growth of single-walled carbon nanotubes at 4 seconds (top left), 10 s (top right), 20 s (bottom left) and 40 s (bottom right). Scale bars represent 1 nanometer. Cobalt (red) and cobalt-carbide (blue) regions of the catalyst nanoparticle show how each region fluctuates during the growth period. (Image: NIST) (click on image to enlarge)
|
|
To build a better catalyst nanoparticle, researchers need a detailed understanding of the chemical pathways involved in the catalytic process, said NIST researcher Renu Sharma. “If you want to make a good catalyst, you want to know why it works and why it doesn’t work, and it’s often changes in the atomic structure of the particle that hold the key to that understanding,” Sharma said.
|
|
To gain that knowledge, she and her colleagues relied on an innovative electron microscope and a high-resolution camera to track in real time the chemical evolution of cobalt nanoparticles as they acted as catalysts for growing single-walled carbon nanotubes. Although cobalt plays an important role in forming the nanotubes, “the question has always been how this catalyst actually works,” said Sharma.
|
|
Sharma and her colleagues, including collaborators from the University of Maryland and Texas A&M University, described their findings in a recent issue of the Journal of Catalysis ("Direct evidence of atomic-scale structural fluctuations in catalyst nanoparticles").
|
|
Scientists have often assumed that a catalyst for making carbon nanotubes functions by attracting gas molecules that participate in the reaction to its surface, where it’s easier to break and forge bonds. But Sharma’s team discovered that in case of the cobalt nanoparticle catalyst, the entire particle is involved, not just its surface.
|
|
|
|
Atomic-resolution video shows that within a single catalyst nanoparticle, the amount of cobalt (blue) and cobalt carbide (magenta)fluctuates as it promoted the growth of single-walled carbon nanotubes (diagonal gray lines emanating from the nanoparticle). The researchers took the video with an environmental transmission electron microscope. (Video: NIST)
|
|
In the experiment, the team used acetylene (C2H2) as a source of carbon to build the nanotubes. The researchers found that some of the carbon, instead of directly making carbon nanotubes at the surface of the cobalt nanoparticle, diffused into the nanoparticle. Inside the nanoparticle, the diffused carbon temporarily formed cobalt carbide (Co2C). This was confirmed by atomic-resolution images taken with an electron microscope, which showed that within a single catalyst nanoparticle, cobalt and cobalt carbide co-existed.
|
|
Eventually, the cobalt carbide decomposed back into cobalt and carbon. Only this carbon contributed to the formation of the nanotubes. The study revealed for the first time that the fluctuating amount of carbon within the catalyst nanoparticles mirrored the fluctuating rate at which the carbon nanotubes grew. Simulations of the molecular process corroborated the finding.
|
|
Now that the team has documented the atomic-scale dynamics of the cobalt catalyst nanoparticles, further studies will be needed to determine how to apply that knowledge to more efficiently form carbon nanotubes, Sharma says. In the meantime, the approach adopted by the researchers—combining real-time, atomic-resolution imaging with molecular dynamics simulations—can be applied to understanding and improving the function of a wide array of catalysts as well as fine-tuning the growth of the structures they help produce.
|

