| Posted: Oct 13, 2017 |
Synthetic 'purple membranes' transform sunlight to hydrogen fuel
(Nanowerk News) Researchers at the U.S. Department of Energy’s Argonne National Laboratory have found a new way to produce solar fuels by developing completely synthetic bionano machinery to harvest light without the need for a living cell.
|
|
The researchers’ technique, reported in ACS Nano ("Cell-Free Synthetic Biology Chassis for Nanocatalytic Photon-to-Hydrogen Conversion") as a “synthetic purple membrane,” is an important step toward producing clean fuels that can help solve global energy challenges.
|
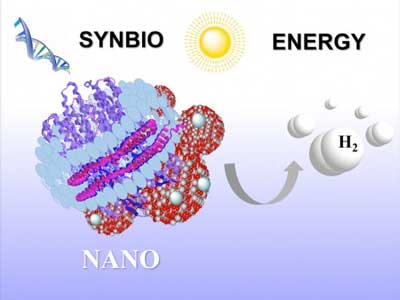 |
| This shows the synthetic purple membrane assembly developed by Elena Rozhkova and fellow Argonne researchers. The assembly, which includes nanodiscs, titanium dioxide and platinum nanoparticles, can transform sunlight into hydrogen fuel. (Image: Argonne National Laboratory)
|
|
These synthetic purple membranes contain tiny discs of organic compounds called lipids, man-made proteins and semiconducting nanoparticles that, when taken together, can transform sunlight into hydrogen fuel.
|
|
“Unlike some other modern approaches, we have been able to use environmentally friendly, cadmium-free materials to make this nanoarchitecture work efficiently under visible light,” said Elena Rozhkova, a scientist at Argonne’s Center for Nanoscale Materials and author of the study.
|
|
Central to the artificial template created by the Argonne researchers is synthetically produced bacteriorhodopsin. This protein is normally found in the membranes of Halobacterium salinarum, an ancient single-celled organism that lives in extreme high-salt conditions such as Utah’s Great Salt Lake and Yellowstone National Park’s hot springs, appearing as purple plumes of water.
|
|
In bacteria, the protein uses energy from visible light to pump protons across the cell membrane, creating an electrical gradient the organism uses to generate and store chemical energy.
|
|
“This synthetic system gives us the ability to reconfigure an ancient biological process for a new and useful application for energy,” Rozhkova added. “In a natural purple membrane, bacteria use bacteriorhodopsin to harvest energy from light. Synthetic purple membranes allow us to use the nanotechnological tools we have created to adapt this to generate energy and serve human needs,” she said.
|
|
“We’re not isolating a natural system to generate energy from sunlight, but rather we are constructing a completely man-made system for designed-protein expression without the need for biological cells, and then combining them with semiconductor particles.”
|
|
Previously, a cell-free protein synthesis platform was used for structural biology and manufacturing proteins for medical applications.
|
|
“This is why we use the nanodiscs — they mimic the biological membrane which normally supports bacteriorhodopsin and enables its function,” Rozhkova said.
|
|
To create the synthetic version of the membrane protein, the researchers used a minimum of key cell elements: the nanodiscs, synthetic DNA that encoded the protein, other biological components needed for protein manufacturing, including amino acids, and also isolated ribosome-protein manufacturing machinery. This led to the successful expression of synthetic bacteriorhodopsin across the nanodiscs.
|
|
“The process of the artificial protein synthesis was visualized with great precision using high resolution scanning probe microscopy,” said Val Novosad, a materials scientist at Argonne.
|
|
Once prepared, the synthetic purple membranes were assembled with nanoparticles of titanium dioxide for hydrogen evolution under visible light. The results reveal the entirely man-made system used the energy from the light to produce hydrogen with similar or even higher efficiency compared to systems based on bacterial purple membrane.
|
|
“When the artificial protein-modified titanium dioxide absorbs the visible light, it uses the energy of the light to generate electrons, which eventually interact with protons on the surface of a co-catalyst to form hydrogen,” said Peng Wang, a former Argonne postdoctoral appointee and another author of the study.
|
|
The study highlights the semiconductor’s ability to harness energy from visible light as opposed to ultraviolet (UV) light, a function central to renewable energy research.
|
|
“Of all the light coming in from sun, only about four percent of it contains UV light, which makes UV light not the best option in terms of energy production. Also, UV light is harmful to the environment,” Wang added.
|

