| Posted: Oct 25, 2017 |
Watching catalysts evolve in 3D
(Nanowerk News) In batteries and fuel cells, certain materials offer needed electrons. Observing and measuring chemical changes in these materials, or catalysts, at the atomic level during oxidation (electron loss) has long been a challenge. Scientists obtained this view of a nickel-cobalt catalyst. They did so by combining multiple advanced electron microscopy techniques.
|
|
Their work (Nature Communications, "Interrogation of bimetallic particle oxidation in three dimensions at the nanoscale") revealed the surprising and complex ways that the tiny catalytic particles lost electrons. They watched the changes the particles undergo on both exterior and interior surfaces.
|
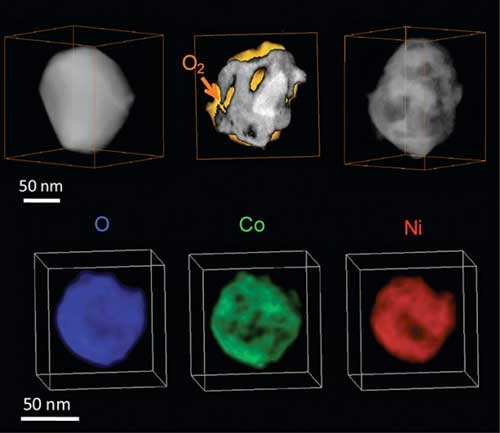 |
| Top row: 3-D evolution of an oxidizing Ni2Co nanoparticle, showing the structure before oxidation (left), during oxidation (middle), and after oxidation (right) (O2 is molecular oxygen). Bottom row: 3-D chemical mapping of the fully oxidized nanoparticle shows the spatial distributions of oxygen (O, blue), cobalt (Co, green), and nickel (Ni, red). (Image: Center for Functional Nanomaterials, Brookhaven National Laboratory)
|
|
Bimetallic (two metal) catalysts are widely employed in batteries and fuel cells. Thus, a full understanding of how they provide electrons is both fundamentally important and of broad technological relevance. Understanding the ways catalysts offer electrons is key to designing these materials for higher performance.
|
|
For example, detailed images of the catalyst working in real time reveal how the particle’s shape changes as it reacts chemically under an oxygen environment. This oxidation controls how the material morphs into the resulting hollow structure.
|
|
Because bimetallic catalysts are widely employed in batteries and fuel cells, a full understanding of their oxidation (electron loss) mechanisms is both fundamentally important and of broad technological relevance.
|
|
To interrogate the structural and chemical changes during oxidation, scientists imaged single Ni2Co catalysts in three dimensions using mass-contrast and chemical sensitive electron tomography. The electron tomography technique retains 3-D information about spatial chemical segregation that is lost in 2-D projected images typically obtained in electron microscopy.
|
|
Moreover, state-of-the-art environmental transmission electron microscopy allowed the scientists to track the catalyst oxidation in situ and in real time. Combining real-time imaging and electron tomography, this study revealed oxidation-induced chemical segregation on both the external and internal surfaces of the catalyst, and elucidated pathways that control and alter the morphology of hollow-structured metal oxides.
|

