| Posted: Dec 06, 2017 |
Mastering tailored design of aluminum nanomaterials
(Nanowerk News) Whether for energy applications or nuclear waste management, industrial processing of aluminum requires understanding its behavior in highly alkaline solutions. Processing slurries and precipitates (typically gibbsite, α-Al(OH)3) from these solutions is aided by controlling the shape of tiny particles that are produced.
|
|
Researchers at the IDREAM Energy Frontier Research Center developed a synthesis route. The scientists devised the route based on simple, rational design principles. With it, the team produced highly uniform gibbsite nanoplates with optimal yield (Crystal Growth & Design, "Fast Synthesis of Gibbsite Nanoplates and Process Optimization using Box-Behnken Experimental Design").
|
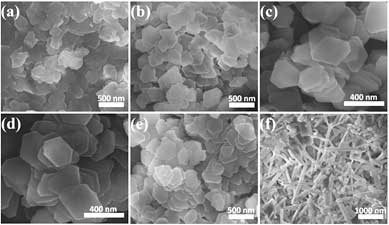 |
| Scanning electron microscopy images of gibbsite synthesized at different pH values: (a) 1; (b) 3; (c) 5; (d) 10; (e) 12; and (f) 13.5. (© ACS)
|
|
Why It Matters: Gibbsite is an important ore of aluminum. The ore is processed on an industrial scale in applications ranging from transportation to energy transmission to high-level radioactive waste treatment. Typical processing is energy intensive. The team's work provides a methodology that is cost-effective and more environmentally friendly than other approaches.
|
|
Gibbsite (α-Al(OH)3) is an important natural and industrial material that is used in a wide variety of energy applications, and is a significant component of some of the high-level nuclear waste stored in large quantities at the Hanford Site, Washington, U.S.A., and at the Savannah River Site, South Carolina, U.S.A.
|
|
Industrial-scale processing of these materials requires an understanding of their behavior in highly alkaline solutions (often called Bayer liquors); processing of slurries and precipitates from these liquors is facilitated by controlling the nanoparticulate gibbsite morphology.
|
|
The IDREAM team has developed a hydrothermal inorganic synthesis route that is based on simple, rational design principles, and leads to highly uniform hexagonal nanoplates within a basal plane diameter range of 200 to 400 nm. Synchrotron-based x-ray absorption spectroscopy for both aluminum and oxygen reveals that the aluminum coordination in the ideal material is a distorted octahedral geometry with oxygen atoms at two, discrete distances from the central aluminum atom.
|

