| Posted: Dec 15, 2017 |
Atoms rearrange in electrolyte and control ion flow under tough conditions
(Nanowerk News) Minerals that make up rocks and soils are thrown out of equilibrium when the chemistry of their surroundings changes. Shifts in pH or the concentration of ions in water make minerals dissolve, grow, or react in other ways. These reactions are influenced by the arrangement of atoms at the interface -- where minerals and water touch.
|
|
Historically, it has been hard to study these structures while reactions are proceeding because the interface is constantly changing, limiting our understanding of how the structures control reaction speed.
|
|
Now, a team led by Dr. Kevin Rosso at DOE's Pacific Northwest National Laboratory (PNNL) achieved the first 3-D view of the atomic structure at the interface of water and the mineral hematite as the reactions occur. The new view showed how the interfacial structure is different while it's reacting, and how these differences might control the flow of ions into the environment (Advanced Functional Materials, "Potential-Specific Structure at the Hematite-Electrolyte Interface").
|
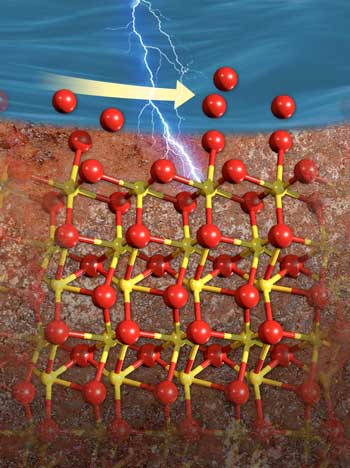 |
| The interface between iron-rich hematite (bottom) and water (top) changes as the surface becomes electrically charged. Oxygen atoms (red) re-arrange on the surface, filling in any spots where oxygen atoms were missing. (Image: Nathan Johnson, Pacific Northwest National Laboratory)
|
|
Why It Matters: Water. Whether it's used to grow crops or is split apart to make hydrogen fuel, accurately modeling water's behavior is vital. This work is the first systematic study of the tiny structures that form at the interface of water and the abundant iron-rich mineral hematite when this interface is far from equilibrium. The research offers key insights about the interface and far-from-equilibrium conditions that influence the interface.
|
|
"These precise measurements will help us build better models of reactions vital to groundwater quality, solar water splitting, and much more," said Dr. Martin McBriarty, a PNNL geoscientist on the project.
|
|
The minerals that make up rocks and soils are often out of equilibrium with their surroundings, especially as environmental conditions change. Minerals respond by dissolving, growing, or transferring charge with their environment. These processes are influenced by the atomic-scale structure at their interface with water. Often the only way to study these structures is when the interface is not changing.
|
|
Researchers at DOE's Pacific Northwest National Laboratory and the University of Chicago obtained the first 3-D view of the atomic structure at the interface of water and the mineral hematite while the hematite is acting as an electrode.
|
|
The team saw how the atoms at the hematite surface and water molecules nearby responded to far-from-equilibrium conditions caused by electrically charging the interface. When the surface was negatively charged, some water molecules became stuck to the surface, while other water molecules became disordered and moved away from the surface.
|
|
What do these structural changes mean? The flow of electrical charge and ions are controlled by the structure while the interface is charged, and the stronger binding of water molecules at the surface might explain why hematite dissolves more slowly than predicted.
|
|
The team's approach to solving these far-from-equilibrium structures could be used to study other interfaces. This is the first systematic study of the atomic- to nano-scale structure of a common mineral-water interface poised far from equilibrium. The research offers a major advance to accurately modeling reactions important to everything from groundwater quality, to energy extraction from the subsurface, to solar water splitting.
|

