| Posted: Jan 10, 2018 |
Atomically controlled gas sensing
(Nanowerk News) An international team of scientists, including those from the LCN at UCL, have solved the stucture of a gas sensing surface of tin dioxide, opening the way to advanced device design (Physical Review Letters, "Structure of the SnO2(110)-(4×1) Surface").
|
|
The basis for the gas sensing as well other applications of tin oxide is the reducibility of the oxide, which allows it to readily and reversibly release oxygen in the presence of reactants such as carbon monoxide or hydrocarbons, and to facilitate adsorption of molecules such as oxygen. The changes in electrical conductivity of this semiconductor resulting from these surface processes are the basis for the material’s gas-sensing characteristics. Because of this, it has long been a goal of fundamental research to characterise the structural, physical, and chemical properties of tin oxide surfaces.
|
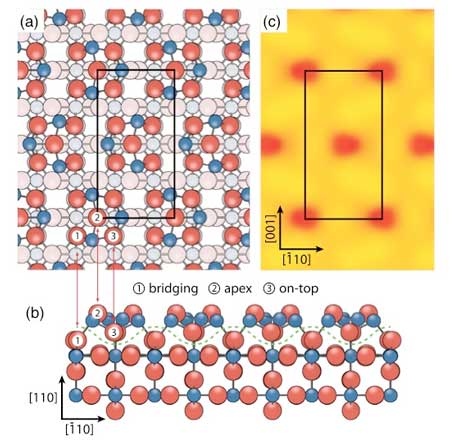 |
| The structure of the tin dioxide surface determined in this work along with a simulation of the scanning tunnelling microscope image. (Image: London Centre for Nanotechnology)
|
|
The (110) plane of tin oxide, the mineral cassiterite, is the most stable under ambient conditions and therefore will be the most abundant termination of the gas sensor. This is the surface that the team investigated with a number of experimental and theoretical techniques, including those available at the Diamond Light Source at Harwell Laboratory.
|
|
The lead author of the work, Lindsay Merte, comments “the tin oxide (4 × 1) termination is one of a handful of gas sensor related surfaces to have its quantitative structure determined. It can now serve as a useful model for fundamental studies of tin oxide surface chemistry and a basis for further studies that should help resolve the complex behavior of the surface under different conditions”
|
|
Considering the importance of tin oxide surface electrical conductivity in gas-sensing applications, it is a natural question whether upon formation of the (4 × 1) termination the surface becomes metallic. To address this, we have evaluated the electronic properties of the upper layers in our structure. This shows that the surface remains semiconducting, which is a necessary requirement for gas sensing.
|

