| Posted: Mar 01, 2018 |
Catalysts: High performance lies on the edge
(Nanowerk News) Platinum is not an abundant element, but it is a popular catalyst. Scientists at Los Alamos National Laboratory synthesized a catalyst made from iron, nickel, and carbon. No platinum. The catalyst had the highest activity to date for a platinum group metal (PGM)-free material used in fuel cells. Experiments showed that the iron-nitrogen reactive sites were predominantly located at the surface-exposed edges, or steps, of the carbon. The reactive sites were not inside the carbon as previously predicted.
|
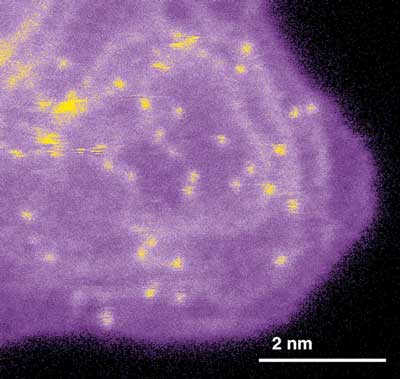 |
| Scanning transmission electron microscopy shows a dispersion of single iron atoms (bright dots) supported on nanostructured carbon (dark purple). The catalytically active nitrogen-coordinated iron atoms are predominantly associated with the edges, or steps, on graphite surface planes (light purple). (Image: David Cullen, Oak Ridge National Laboratory)
|
|
Atomic-level microscopy offers insight into the success of a PGM-free alternative. It’s based on cheap, abundant elements—iron, nitrogen, and carbon. The insights are guiding future research and development of high-performance PGM-free catalysts.
|
|
In fuel cells, promotion of the reaction of oxygen and hydrogenat the cathode (the oxygen reduction reaction) currently requires the use of expensive PGM catalysts. The slow reaction of oxygen and hydrogen at the cathode limits the performance of current fuel cells with catalysts based on rare, expensive PGMs.
|
|
To reduce costs, scientists at Los Alamos National Laboratory developed a new PGM-free catalyst by heating carbon- and nitrogen-containing precursors—polyaniline (PANI) and cyanamide (CM)—with iron to synthesize a novel catalyst (Science, "Direct atomic-level insight into the active sites of a high-performance PGM-free ORR catalyst").
|
|
To understand why the new catalyst performed so well, the researchers relied on the novel electron microscopy resources and expertise at Oak Ridge National Laboratory, where a low-voltage aberration-corrected scanning transmission electron microscope equipped with electron energy loss spectroscopy identified the locations and bonding of the individual atomic elements comprising the catalyst.
|
|
Previous studies predicted iron coordinated by four nitrogen atoms embedded in graphene would be the optimal active site. However, microscopy showed that the iron-nitrogen sites were in fact predominantly associated with exposed basal plane edges, or steps, of the nanocrystalline carbon support (known as edge-hosted sites) rather than being embedded within graphene (bulk-hosted sites), as predicted by previous theoretical work.
|
|
The use of two precursors, PANI and CM, during catalyst synthesis also resulted in a wide range of pore sizes that increased the exposed surface area of the carbonaceous support and increased access to the active sites—explaining the remarkable activity approaching that of PGM-based catalysts.
|

