| Posted: Mar 30, 2018 |
What a mesh
(Nanowerk News) A team of scientists from across the U.S. has found a new way to create molecular interconnections that can give a certain class of materials exciting new properties, including improving their ability to catalyze chemical reactions or harvest energy from light.
|
|
In a new study (Nature Materials, "A Molecular Cross-Linking Approach for Hybrid Metal Oxides"), researchers at the U.S. Department of Energy’s (DOE) Argonne National Laboratory, the University of California-Los Angeles, the University of California-Santa Barbara, Purdue University and the University of Oregon have developed a method to create linked networks of metal oxides that could have interesting catalytic or electronic properties.
|
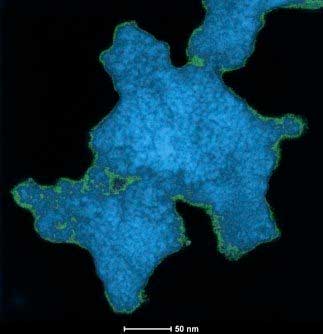 |
| This shows electron microscopy of cross-linked titania nanoparticles with boron-based clusters. Argonne researchers helped create a method to build these networks. (Image: Alexander Spokoyny, UCLA)
|
|
Metal oxides are of interest to scientists because of their unique electronic and chemical properties. Some, like titanium dioxide, are commonly used in photovoltaic and photocatalytic applications because of their ability to absorb light.
|
|
The key to forming these metal oxide networks is boron, which when annealed with metal oxides leads to the formation of thermally robust and stable interconnected clusters that act as strands of glue that connect a metal oxide web.
|
|
“This glue has the ability to be a key component of the entire reactive system, changing the properties that the metal oxides had on their own,” said Alexander Spokoyny, a chemist at UCLA.
|
|
The formation of the boron-metal oxide network provides a launching point for future studies of different materials that could combine their own natural properties with the added advantage of a similar “cross-linked” structure.
|
|
“We want to know, for instance, if we can transfer our knowledge of this mesh to a material like silicon dioxide. The photocatalytic properties of these materials are extraordinary compared to titanium dioxide,” said Argonne chemist Max Delferro.
|
|
In the future, the researchers seek to design a way to create precisely tailored materials by perfecting how the interconnecting clusters of boron “glue” are interspersed within the metal oxide. “If we can stitch in these molecules exactly where we want them to be, it will give us a powerful ability to make and understand hybrid materials with a wide range of uses,” Spokoyny said.
|
|
Because these materials are so new, the researchers believe they have a great deal of untapped potential. “We’re not claiming mission fully accomplished by any means; there are still parts of the chemistry that we don’t fully understand and appreciate,” Delferro said.
|
|
The research team included Argonne chemist Karena Chapman, who works at the laboratory’s Advanced Photon Source (APS), a DOE Office of Science User Facility. Chapman and Spokoyny met when they were named to Chemical and Engineering News’s “Talented Twelve” list in 2016, and established the collaboration that led to the research.
|
|
According to Chapman, a member of the Structural Sciences Group in the APS X-ray Science division, the structural characterization of the material involved the use of X-ray pair distribution function analysis carried out at the APS, which gives local structural information about the relative atom positions.
|
|
Chapman, Delferro and Spokoyny noted that the efforts of the research team to produce and analyze this new material were just as interconnected as the discovered hybrid material itself. “There are cross-linkages at both the molecular and the human level,” Delferro said. “This work proves that we work better and are stronger when we’re connected.”
|

