| Posted: Apr 11, 2018 |
Sensing interactions between molecules
(Nanowerk News) In a recent study published in the scientific journal Nature Nanotechnology ("Quantitative assessment of intermolecular interactions by atomic force microscopy imaging using copper oxide tips"), physicists and chemists of the University of Münster (Germany) describe an experimental approach to visualising structures of organic molecules with exceptional resolution. The key to this newly developed microscopic method is the high stability of a particularly sharp and atomically defined probe tip.
|
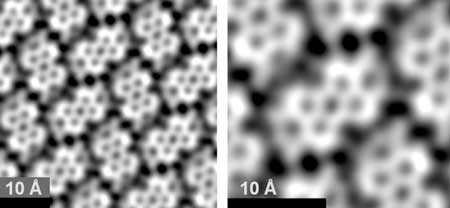 |
| Overview image of a self-organized molecular network. Right: Enlarged image extracted from the left image. It shows a single molecule (center) surrounded by six partly visible ones. The faint lines between the molecules indicate dominant sites of the molecule-molecule interactions. (© Springer Nature)
|
|
The new method which can be used to image the structural and chemical properties of organic molecules with extreme precision was developed by physics researchers in the labs of the Center for Nanotechnology (CeNTech) at the University of Münster.
|
|
The experiment is based on atomic force microscopy where sample surfaces are scanned with the apex of a needle-like probe. As the lead author of the study Dr. Harry Mönig explains: "Our special technique involves a copper-based probe tip which is passivated by a single oxygen atom at the tip termination."
|
|
Here, passivation means that the oxygen atom reduces undesired interaction between the atoms of the tip and the atoms in the molecules under investigation. This greatly increases the imaging resolution. In contrast to previous methods, the bond between the oxygen atom at the tip and copper base is particularly strong, thereby reducing imaging artefacts to a minimum.
|
|
Prof. Dr. Harald Fuchs, co-author of the study, emphasises: "The potential of the new method is considerable as it allows us to investigate bonding structures of molecular networks with exceptional accuracy."
|
|
Providing fundamental insights into the interactions between molecules is important for the development of new so-called nanostructured materials. Such materials take advantage of the fact that very small deviations on the nanoscale can significantly alter the material properties. The difference between diamonds and graphite is a well-known example of such nanoscale deviations. Although both consist of pure carbon, diamond is extremely hard whereas graphite is comparatively soft. Only the structural arrangement and bonding between the carbon atoms are different.
|

