| Posted: Apr 13, 2018 |
Seeing how next-generation batteries power-up
(Nanowerk News) Next-generation batteries based on magnesium rather than lithium ions hold promise to be more efficient, safer, and cheaper. One electrode is metallic magnesium. During discharge, magnesium moves from this electrode to the positive electrode (cathode). But where does the magnesium go when it enters the cathode?
|
|
Using sophisticated electron microscopy, scientists could see these atoms—even at low concentrations. They discovered that magnesium atoms are inserted into the structure of the cathode material at predicted locations (Chemistry of Materials, "Direct investigation of Mg intercalation into the orthorhombic V2O5 cathode using atomic-resolution transmission electron microscopy").
|
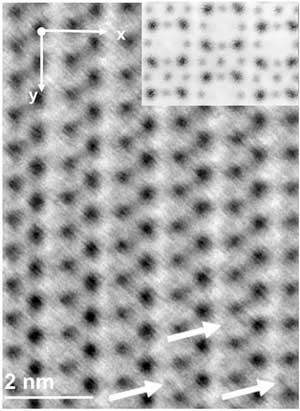 |
| Sophisticated electron microscopy allowed scientists to directly visualize magnesium going into an advanced battery electrode. During discharge, magnesium atoms move from one electrode (magnesium metal) to a positive electrode (vanadium oxide in the image), called the cathode. The high-resolution image shows the atomic arrangement in the cathode after discharge. The image shows the columns of vanadium (black) and oxygen (grey) atoms. The arrows identify several locations where magnesium has migrated within the atomic structure. (© ACS)
|
|
Magnesium ions are doubly charged compared to singly charged lithium ions. Thus, magnesium-based batteries could store more energy. This performance requires the development of a cathode material that can accommodate the magnesium migration during discharge—a difficult challenge. The study provides details about how and where magnesium atoms are stored in a cathode structure. These insights could lead to improved design “rules” for superior materials that enable magnesium-based batteries.
|
|
Batteries based on magnesium metal as the negative electrode (anode) promise much higher energy density compared to lithium-ion systems and are, at the same time, potentially safer and more cost-effective. A roadblock to the development of these batteries is the lack of an effective cathode (positive electrode) material.
|
|
To be an effective cathode for this battery architecture, the material needs to be able to accommodate magnesium ions into its structure (a process called intercalation) when the battery discharges and then release the ions during charging.
|
|
Previous experimental studies have reported reversible magnesium intercalation into some cathode materials based on indirect electrochemical data. However, today’s transmission electron microscopy techniques provide unique capabilities to directly image magnesium intercalation and quantify the reactions within the cathode material.
|
|
In this study, scientists carried out in-depth research of magnesium insertion into a cathode composed of vanadium oxide. They combined aberration-corrected scanning transmission electron microscopy imaging, electron energy-loss spectroscopy, and energy-dispersive X-ray spectroscopy analysis.
|
|
They compared the results from two vanadium oxide samples: (1) an electrochemically cycled vanadium oxide cathode coupled with a magnesium metal anode and (2) a chemically synthesized vanadium oxide material with magnesium already in it.
|
|
Results suggest that the electrochemically cycled vanadium oxide cathode did contain magnesium, which indicates true intercalation is occurring in this material. That process also resulted in a local formation of a theoretically predicted phase of vanadium-magnesium oxide. This phase is different from the chemically synthesized sample, and the levels of magnesium in the structure were lower than predicted.
|
|
This research furthered our fundamental understanding of how magnesium atoms are stored. These insights could lead to the design of superior cathode materials.
|

