| Posted: Apr 17, 2018 |
A novel, water-stable metal-organic framework for selective CO2 adsorption
(Nanowerk News) Researchers of the University of Amsterdam’s Research Priority Area Sustainable Chemistry have developed a novel porous material capable of selectively adsorbing CO2. In the journal Inorganic Chemistry Frontiers ("Selective CO2 adsorption in water-stable alkaline-earth based metal-organic frameworks") they present their design of a water-stable metal-organic framework (MOF) that according to team leader Dr Stefania Grecea can pave the way to cost-effective separation of CO2 from power plant emissions.
|
|
Climate change is correlated with increasing CO2 emissions as a result of the combustion of fossil fuels in power stations, vehicles, factories and homes. One way to keep the atmospheric CO2concentration within acceptable levels is by selectively removing it from power plant emissions. The physical adsorption of CO2 using porous materials provides an economically viable route for such CO2 capturing. It requires less energy for the regeneration of adsorbents in comparison to the currently used chemisorption methods.
|
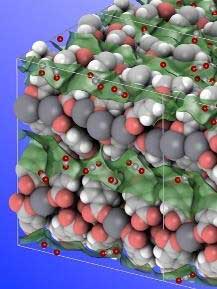 |
| Molecular structure of the novel calcium-based MOF. Small CO2 molecules can be seen adsorbed within the channels (green transparent) of the microporous material. (Image: HIMS)
|
|
At the Research Priority Area Sustainable Chemistry of the University of Amsterdam (UvA), Dr Stefania Grecea develops synthetic strategies for making porous materials that enable efficient adsorptive separations.
|
|
In the journal Inorganic Chemistry Frontiers, Grecea and co-workers now present a water-stable metal-organic framework that selectively adsorbs CO2 from a CO2/N2 mixture. The research was performed by PhD student Yiwen Tang in collaboration with Dr David Dubbeldam of UvA's Computational Chemistry group, Dr Simon Teat of the Lawrence Berkeley National Laboratory (USA), and Prof. Anastasios Tasiopoulos of the University of Cyprus.
|
Metal-organic frameworks
|
|
Metal-organic frameworks (MOFs) are crystalline microporous materials that exist of metal ions (or clusters of metal ions) linked by organic ligands. MOFs are very promising materials for gas separation since their pore surfaces can be easily functionalised to tune the interaction with various gas molecules. They also have very high surface areas which provide for optimal selectivity and capacity. Moreover, MOFs offer the right balance between high adsorption capacity, operational life time and energy requirement for regeneration.
|
|
The UvA team designed a series of MOFs using a flexible organic linker in combination with alkaline metal ions, which are abundantly available, lightweight and low-cost. They show that when the guest molecules are removed, the three-dimensional structure of these MOFs shrinks. In the case of calcium based materials, this specific behaviour results in large diffusional resistances towards N2 over CO2 molecules.
|
Selective and water-stable
|
|
Experimental studies performed by Yiwen Tang and theoretical calculations performed by Dr David Dubbeldam confirmed a very good CO2/N2 separation selectivity that is substantially higher than what has been reported for other MOFs. Adding to this, the specific design of the organic linker protects the calcium ions from being attacked by water molecules. Therefore, the new MOF materials also have a very high humidity stability.
|
|
According to Dr Grecea, the study opens new avenues in developing low-cost and non-toxic materials, not only for CO2capture but also for other gas separations. Currently her team is further improving the water stability of the novel MOFs as well as developing general synthetic routes towards stable alkaline-earth MOFs for a broad range of molecular separations.
|

