| Posted: Apr 17, 2018 |
How does a molecule vibrate when you 'touch' it?
(Nanowerk News) Physicists from the University of Regensburg (Germany), the Kanazawa University (Japan) and the Linnaeus University in Kalmar (Sweden) have studied the vibrations of a carbon monoxide molecule (CO, black and red ball in Figure below) that is bonded on a copper surface under the influence of an external force field exerted by the tip of a scanning probe microscope (PNAS, "Vibrations of a molecule in an external force field").
|
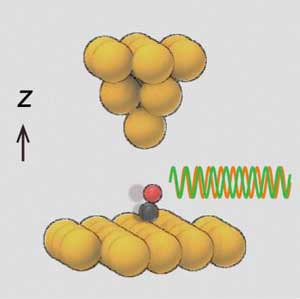 |
| The probe tip of a scanning probe microscope alters the vibrational frequency of the molecule and allows to determine the strength of the bonds. (Image: Norio Okabayashi)
|
|
The measurements were conducted at the University of Regensburg using combined scanning tunneling microscopy, scanning tunneling spectroscopy and atomic force microscopy at liquid helium temperatures and ultrahigh vacuum.
|
|
The CO molecule bonds with the carbon atom to the copper underneath and stands upright on the surface such that the oxygen atom points away from the surface.
|
|
The CO molecule can oscillate just like an inverted pendulum. The vibration of a molecule on a surface contains critical information on the bond of the molecule with the surface, which is crucial for understanding surface phenomena and for technologically important processes such as catalysis and epitaxial growth.
|
|
As expected, the force that originates from the probe tip (pointed object from above in Figure) changes the vibrational frequencies – attractive forces increase the oscillation frequency, and repulsive interactions decrease the oscillation frequency.
|
|
The data revealed that the strength of the bond between carbon monoxide and copper was decreasing as the probe tip pulled the molecule away from the surface, marking the direct observation of the weakening of a single atomic bond by an external influence.
|
|
The result is important as chemical reactions often evolve by loosening an existing bond before forming a new one.
|

