| Posted: Jun 22, 2018 |
Sense like a shark: Saltwater-submersible films
(Nanowerk News) Just like a shark, a new film detects minute changes in nearby electric fields while immersed in a harsh environment. The thin film contains samarium, nickel, and oxygen (SNO). When submerged in water and exposed to an electric bias, the film undergoes a reproducible and reversible change in its structure. That is, it incorporates protons into the lattice. This tiny change can be sensed as a voltage.
|
|
Electrical resistance of the SNO sensor can be tuned when a small (millivolt level) electrical bias potential is applied. This property may aid the monitoring of electrical signals from maritime vessels and sea creatures.
|
|
Designing materials for sensors that function at peak performance in relatively harsh environments, such as saltwater, is of interest to technologies ranging from energy utilization and ocean monitoring to biological applications.
|
|
One primary challenge in sensor design is to retain the structure and activity of the material as it interacts dynamically with the surrounding environment. Materials that respond to mild stimulation through temporary changes in their structure—or phase transitions—and that amplify signals could open up new avenues for sensing.
|
|
Scientists discovered an electric-field-driven and reversible structural phase change in a thin samarium nickelate (SmNiO3) film while submerged in water (Nature, "Perovskite nickelates as electric field sensors in salt water").
|
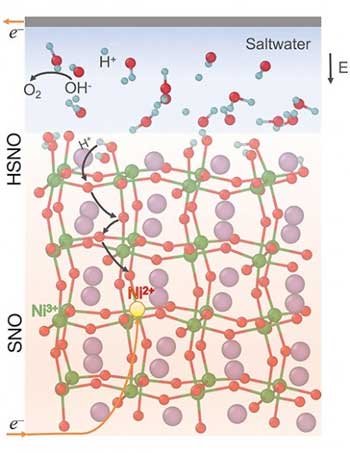 |
| Saltwater helps a reversible phase transition occur in samarium nickelate (SNO). Under electrical bias, protons (H+) enter and diffuse into the SNO lattice (forming HSNO) with electron (e-) transfer from the counter electrode happening at the same time. This voltage-bias induced phase change results in exceptional changes in electrical resistivity and enables detection of sub-volt electric potentials in water. The samarium, nickel, hydrogen, and oxygen atoms are shown as purple, green, blue, and red spheres, respectively. (Image: Badri Narayanan, Argonne National Laboratory)
|
|
The discovery was made by a team of users of the Center for Nanoscale Materials (CNM), Advanced Photon Source (APS), National Energy Research Scientific Computing Center, and the Argonne Leadership Computing Facility (ALCF) from Purdue University.
|
|
SNO is a commonly studied quantum material—materials with electronically cooperative behavior that can’t be explained by individual electron properties—because it is stable in salt water, does not corrode, and allows the exchange of protons with the surrounding water at room temperature.
|
|
Numerical calculations of the molecular SNO-water interactions were performed on computing resources at the CNM and the ALCF to explain the sensing mechanism in detail. X-ray reflectivity and absorption spectroscopy were performed at the APS.
|
|
In addition to performing as both self-regulating heating elements and pH sensors, devices made of this material can detect very small electric potentials in salt water. Therefore, such devices could be used in oceanic environments for monitoring electrical signals from maritime vessels and sea creatures.
|

