| Posted: Jun 26, 2018 |
Twisted meta-molecules as they really are
(Nanowerk News) Physicists at the University of Bath have devised a new and highly sensitive method to truly test the chirality of a material, eliminating the risk of false positives from competing effects (ACS Nano, "Second-Harmonic Generation Optical Rotation Solely Attributable to Chirality in Plasmonic Metasurfaces").
|
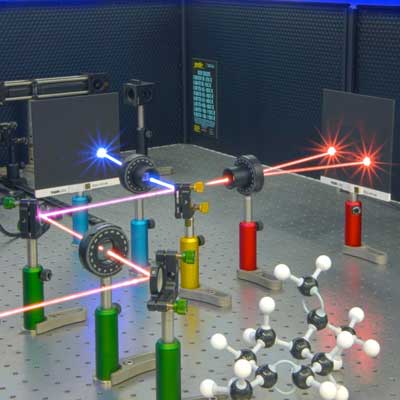 |
| A laser beam changed from red to blue by interacting with chiral molecules. (Image: Ventsislav Valev and Joel Collins)
|
|
Chiral molecules exist in different forms, even when they are made of the same atoms, those atoms can be arranged differently - twisting one way or another. This crucial difference can affect the properties of the molecules and has applications in fields such as telecommunications, nanorobotics, pharmaceuticals and industrial chemicals.
|
|
However, because of the nanoscopic nature of many of these molecules and materials it can be difficult for scientists to be certain they are working with chiral molecules of a particular twist (known as 'handedness'). Some tests which are used can produce false positives, meaning scientists could be left working with the wrong samples.
|
|
The University of Bath team, working with colleagues at the Max Planck Institute for Intelligent Systems in Germany, demonstrated a method to separate the chirality of a substance from sources of false positives. In order to achieve this they used the way light interacts with artificial molecules (known as 'meta-molecules') that were made of tiny gold helices.
|
|
The team directed a powerful laser beam at samples of chiral meta-molecules, which caused it to change colour: from red to blue. Because the sample chirality imprints a twist on light, the blue light was also twisted: 45° left or right, depending on the sample handedness. Previously, false positives (such as anisotropy) have been known to affect chiral optical measurements.
|
|
Joel Collins, who conducted the experiments said: "Some other techniques can produce a similar effect but without this being due to the chirality which can be misleading. By being able to rotate the sample and retain the optical effects we have a true test of chirality, and you can be sure what you're seeing is due to chirality and not some other property."
|
|
Dr Ventsislav Valev who led the research said: "Meta-molecules are scientifically very exciting. Our demonstration of this effect, free from anisotropy contributions, is a first in the research field. Plus, we were astounded by how large it was. A 45° twist usually requires travelling through 5 cm of concentrated sugar syrup. Our material is half a million times thinner."
|

