| Jul 03, 2018 | |
High performance nitride semiconductor for environmentally friendly photovoltaics(Nanowerk News) A Tokyo Institute of Technology research team has shown copper nitride acts as an n-type semiconductor, with p-type conduction provided by fluorine doping, utilizing a unique nitriding technique applicable for mass production and a computational search for appropriate doping elements, as well as atomically resolved microscopy and electronic structure analysis using synchrotron radiation (Advanced Materials, "High-Mobility p-Type and n-Type Copper Nitride Semiconductors by Direct Nitriding Synthesis and In Silico Doping Design"). |
|
| These n-type and p-type copper nitride semiconductors could potentially replace the conventional toxic or rare materials in photovoltaic cells. | |
 |
|
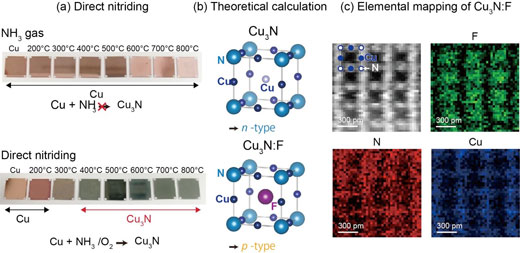
| (a) This is copper and Copper Nitride. (b) Theoretical Calculation for P-type and N-type Copper Nitride. (c) Direct Observation of Fluorine Position in Fluorine-doped Copper Nitride. (a) An image of thin film copper plates before and after reacting with ammonia and oxygen. Copper metal has been transformed to copper nitride. (b) Copper insertion for an n-type semiconductor and fluorine insertion for a p-type semiconductor. (c) Nitrogen plotted in red, fluorine in green, and copper in blue. Fluorine is located at the open space of the crystal as predicted by the theoretical calculation. (© Advanced Materials) (click on image to enlarge) | |
| Thin film photovoltaics have equivalent efficiency and can cut the cost of materials compared to market-dominating silicon solar panels. Utilizing the photovoltaic effect, thin layers of specific p-type and n-type materials are sandwiched together to produce electricity from sunlight. | |
| The technology promises a brighter future for solar energy, allowing low-cost and scalable manufacturing routes compared to crystalline silicon technology, even though toxic and rare materials are used in commercialized thin film solar cells. A Tokyo Institute of Technology team has challenged to find a new candidate material for producing cleaner, cheaper thin film photovoltaics. | |
| They have focused on a simple binary compound, copper nitride that is composed of environmentally friendly elements. However, growing a nitride crystal in a high quality form is challenging as history tells us to develop gallium nitride blue LEDs. | |
| Matsuzaki and his coworkers have overcome the difficulty by introducing a novel catalytic reaction route using ammonia and oxidant gas. This compound, pictured through the photograph in figure (a), is an n-type conductor that has excess electrons. On the other hand, by inserting fluorine element in the open space of the crystal, they found this n-type compound transformed into p-type as predicted by theoretical calculations and directly proven by atomically resolved microscopy in figures (b) and (c), respectively. | |
| All existing thin film photovoltaics require a p-type or n-type partner in their makeup of a sandwich structure, requiring huge efforts to find the best combination. P-type and n-type conduction in the same material developed by Matsuzaki and his coworkers are beneficial to design a highly efficient solar cell structure without such efforts. This material is non-toxic, abundant, and therefore potentially cheap--ideal replacements for in use cadmium telluride and copper indium gallium diselenide thin film solar cells. | |
| With the development of these p-type and n-type semiconductors, in a scalable forming technique using simple safe and abundant elements, the positive qualities will further bring thin film technology into the light. |
| Source: Tokyo Institute of Technology | |
|
Subscribe to a free copy of one of our daily Nanowerk Newsletter Email Digests with a compilation of all of the day's news. |
