| Jul 06, 2018 | |
Scanning tunneling microscopy reveals molecular dissociation induced by localized surface plasmons(Nanowerk News) Using a special type of electron microscope, RIKEN researchers have observed a single molecule sitting on a metal surface split into two due to electron vibrations set off by the interaction of light with the microscope tip (Science, "Real-space and real-time observation of a plasmon-induced chemical reaction of a single molecule"). This observation has revealed the mechanism of this light-induced catalytic reaction for the first time. |
|
 |
|
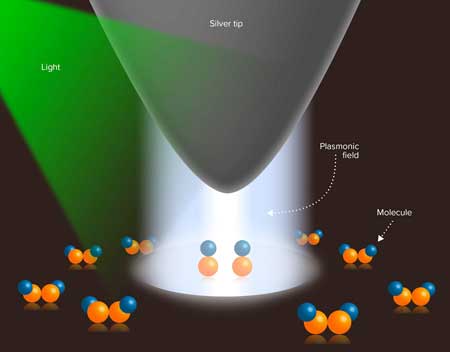
| Schematic representation of the experimental setup for the direct observation of plasmon-induced molecular dissociation. The silver tip of a scanning tunneling microscope is positioned about 1 nanometer above a metal surface on which dimethyl disulfide molecules are adsorbed. The irradiated plasmonic field then initiates dissociation of the molecule. (Image: RIKEN Cluster for Pioneering Research) | |
| The interaction of light with metal nanoparticles can be used to catalyze chemical reactions. Such photocatalysts are attractive from an environmental perspective since chemical reactions could be assisted by sunlight. But to design more-efficient photocatalysts for chemical reactions requires a deep understanding of the catalytic process at the molecular level. | |
| Light can excite collective oscillations of electrons on the surfaces of metal nanoparticles. Known as localized surface plasmons (LSPs), these oscillations can be used to efficiently transfer energy to molecules adsorbed on their surface and thereby trigger reactions. However, the mechanism for this transfer has been unclear because the reaction occurs on such a small scale. In particular, it has not been clear whether the light-induced LSPs interact directly with the molecules or whether the LSPs decay and generate energetic electrons (‘hot electrons’), which then interact with the molecules. | |
| Previous studies have favored the indirect mechanism via hot electrons. “The indirect hot-electron transfer has been put forward as the mechanism for the plasmon-induced break up of oxygen and hydrogen molecules on metal surfaces,” notes Emiko Kazuma of the RIKEN Cluster for Pioneering Research. “However, this proposal arises from several indirect observations and thus is controversial. | |
| Now, by using a scanning tunneling microscope to observe plasmon-induced rupture of a single molecule of dimethyl disulfide (C2H6S2) on silver and copper surfaces (Fig. 1), Kazuma and her co-workers have found that LSPs can directly interact with the adsorbed molecule. | |
| Light excited LSPs in the nanogap between the silver tip of the scanning tunneling microscope and the metallic surface. These LSPs then directly transferred their energy to molecules absorbed on the metal surface, exciting electrons in the molecule, which caused the molecule to split into two due to the breaking of the bond between its two sulfur atoms. | |
| Kazuma and co-workers found that the mechanisms of plasmon-induced reaction, direct or indirect, can in principle be guided by tuning how strongly an adsorbed molecule is bound to the catalyst surface. Strongly adsorbed molecules such as oxygen and hydrogen would dissociate through an indirect hot-electron transfer mechanism, whereas weakly adsorbed molecules prefer a direct intramolecular excitation mechanism, as in the case of dimethyl disulfide. | |
| “These findings provide deep insights into the interaction between LSPs and molecules at metal surfaces for designing efficient plasmon-induced photocatalysis in a highly controlled fashion,” Kazuma comments. |
| Source: RIKEN | |
|
Subscribe to a free copy of one of our daily Nanowerk Newsletter Email Digests with a compilation of all of the day's news. |
