| Aug 03, 2018 | |
Assembly of fluctuating molecules in artificial cell membrane(Nanowerk News) Lipids and membrane proteins existing in cell membranes, which locates at the outermost layer of cells, are responsible for recognizing extracellular environments and transferring that information inside the cell. |
|
| Due to their deep relation to infection of bacteria and viruses, immunological response and neural transmission, they are important research topics in the fields of biology, medicine and drug development. | |
| In the reaction process of both external recognition and signal transfer, the formation of two-dimensional aggregates of lipids with bulky hydrophilic groups, such as sugar chains or inositol rings, in cell membranes are considered necessary. | |
| Small aggregates of up to 10 molecules are called clusters, while aggregates with more molecules and further growth are called domains. | |
| Lipids are amphiphilic molecules derived from organisms, and have both hydrophilic and hydrophobic properties within their molecules. Many past studies have shown that interactions at the hydrophobic part, such as the phase transition and miscibility of hydrocarbon chains, play an important role in domain formation in lipid bilayer membranes. | |
| On the other hand, interactions at the hydrophilic part of lipids have not been widely researched, with many factors still remaining unclear. Interactions become complicated because of repulsion occurring through the fluctuation of hydrophilic properties in the water, particularly at bulky hydrophilic parts like sugar chains. | |
| The repulsion caused by such fluctuation also affects measurements via atomic force microscopy (AFM) that can detect even the tiniest amount of force. | |
| A research team helmed by Ryugo Tero, Associate Professor in Toyohashi University of Technology, has used fluorescence microscopy and AFM to examine in detail artificial lipid bilayer membranes containing lipids modified by the hydrophilic polymer, polyethylene glycol (PEG). | |
 |
|
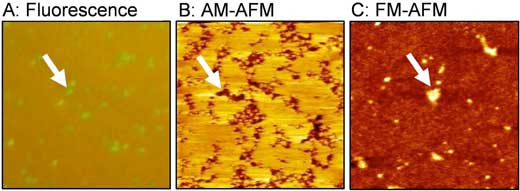
| Figure 1: These are lipid bilayer domains rich in polyethylene glycol (PEG)-modified lipid. (A) Fluorescence image, (B) amplitude modulation (AM) AFM topography, and (C) frequency modulation (FM) AFM topography. (Image: Toyohashi University of Technology) (click on image to enlarge) | |
| The results revealed that two types of aggregates, clusters and domains, form depending on the concentration of PEG-modified lipids, and that the there is almost no fluidity in the domains that appear due to high concentration (Langmuir, "Morphology and Physical Properties of Hydrophilic-Polymer-Modified Lipids in Supported Lipid Bilayers"). | |
| These aggregates are not formed through the interaction of lipids' hydrophobic part, but through the interaction of their hydrophilic part. Interestingly, when observed with AFM, the PEG-modified lipid domains that should have been bulky, were observed at a lower level than the surroundings (Figure 1B). | |
| Associate Professor Tero implemented a joint experiment with Professor Takeshi Fukuma in Kanazawa University about the reasons for this. By utilizing frequency modulation AFM (FM-AFM), and accurately controlling the force between the sample and the probe, they were able to observe the PEG-modified lipid domain at a higher level than the lipid membrane area, without the application of any amount of force (Figure 1C). | |
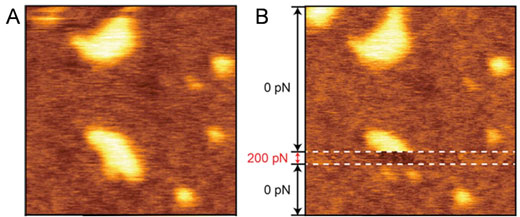
| Since repulsions will change due to the fluctuation of hydrophilic polymer chains dependent on the force applied, it has been found that in general, a reverse image of the true three-dimensional structure inevitably appears at the conditions for amplitude modulation AFM (AM-AFM) observation (Figure 2). | |
 |
|
| This is force-dependent topography of the PEG-lipid-rich domain. (Image: Toyohashi University of Technology) | |
| "In order to establish an experimental method for examining glycolipids' aggregate state and function, we utilized PEG-modified lipids that are easy to obtain at the very beginning. We struggled to find the most suitable conditions for sample preparation and AFM observation of the lipid bilayer membrane containing PEG-modified lipids. | |
| The results differed greatly in comparison to expectations, especially due to the fact that the recessed areas grew with the increase in concentration of PEG-modified lipids. Thinking there might have been a mistake, we repeated the experiment and confirmed its reproducibility. | |
| Intuitively, it may seem unlikely that the region with bulky molecules appears lower with AFM, but when the assembled state of the polymer and the basic principles of AFM are considered, this is actually very reasonable. When the concave-convex properties of the surface reversed after switching to FM-AFM, the joint experiment with Kanazawa University reached its peak and we shouted 'Eureka!'" So explains the main author, Yasuhiro Kakimoto, currently in the doctoral course (Enrolled in the Program for Leading Graduate School organized by the Ministry of Education, Culture, Sports, Science and Technology). | |
| The research team leader, Associate Professor Ryugo Tero said "In order to understand the functions of biomolecules at a molecular level, it is vital to comprehend the appearance of soft molecules, such as lipids and proteins, fluctuating in the water. | |
| In fact, some evidence from experiments areas containing many glycolipids being observed at a lower level with AM-AFM has been obtained for approximately 10 years in several systems. Although repulsion due to fluctuation of hydrophilic parts was only a hypothesis, this study has confirmed its validity. The crucial achievements in this study were the results gained by utilizing Professor Fukuma's state-of-the-art FM-AFM instrument (Figure 2), which lead to this joint research accomplishing magnificent results." | |
| The principle of domain formation due to interactions with hydrophilic polymer chains identified as a result of this research has been found to share commonalities with glycolipids in the cell membrane. Our research team is of the opinion that this principle will assist with the understanding of the mechanism of cell recognition and signal transfers related with the aggregate state of glycolipids and membrane proteins. | |
| Furthermore, the findings from the experiments, in which bulky objects can appear sunken-in depending on the conditions, are vital to many researchers analyzing biological molecules underwater through atomic force microscopy. | |
| In addition, PEGs have the effect of suppressing nonspecific adsorptions such as proteins, etc., and can also be utilized in bio-interfaces and drug delivery. The formation and force response of PEG-rich clusters and domains are expected to have a pervasive effect also in these fields. |
| Source: Toyohashi University of Technology | |
|
Subscribe to a free copy of one of our daily Nanowerk Newsletter Email Digests with a compilation of all of the day's news. |
