| Sep 07, 2018 | |
Nanoparticles form supercrystals under pressure(Nanowerk News) Self-assembly and crystallisation of nanoparticles (NPs) is generally a complex process, based on the evaporation or precipitation of NP-building blocks. Obtaining high-quality supercrystals is slow, dependent on forming and maintaining homogenous crystallisation conditions. Recent studies have used applied pressure as a homogenous method to induce various structural transformations and phase transitions in pre-ordered nanoparticle assemblies. |
|
| Now, in work recently published in the Journal of Physical Chemistry Letters ("Pressure-Stimulated Supercrystals Formation in Nanoparticle Suspensions"), a team of German researchers studying solutions of gold nanoparticles coated with poly(ethylene glycol)- (PEG-) based ligands has discovered that supercrystals can be induced to form rapidly within the whole suspension. | |
 |
|
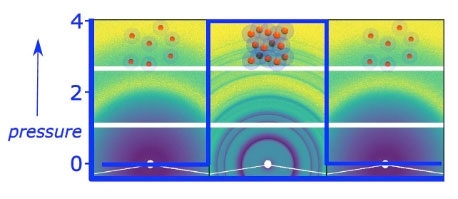
| Fig. 1: 2D SAXS patterns of PEG-coated gold nanoparticles (AuNP) with 2 M CsCl added at different pressures. Left: 1 bar; Middle: 4000 bar; Right: After pressure release at 1 bar. The scheme on top illustrates the structural assembly of the coated AuNPs at different pressures: At 1 bar, the particle ensemble is in an amorphous, liquid state. Upon reaching the crystallization pressure, face-centred cubic crystallites are formed by the AuNPs. After pressure release, the AuNPs return to the liquid state. | |
| Over the last few decades, there has been considerable interest in the formation of nanoparticle (NP) supercrystals, which can exhibit tunable and collective properties that are different from that of their component parts, and which have potential applications in areas such as optics, electronics, and sensor platforms. | |
| Whilst the formation of high-quality supercrystals is normally a slow and complex process, recent research has shown that applying pressure can induce gold nanoparticles to form supercrystals. Building on this and the established effect of salts on the solubility of gold nanoparticles (AuNP) coated with PEG-based ligands, Dr Martin Schroer and his team carried out a series of experiments investigating the effect of varying pressure on gold nanoparticles in aqueous solutions. | |
| They made an unexpected observation – when a salt is added to the solution, the nanoparticles crystallise at a certain pressure. The phase diagram is very sensitive, and the crystallisation can be tuned by varying the type of salt added, and its concentration. | |
| The team used Small Angle X-ray Scattering (SAXS) on beamline I22 to study the crystallisation in situ with different chloride salts (NaCl, KCl, RbCl, CsCl). | |
| As Dr Schroer explains, I22 is one of the few beamlines to offer a high-pressure environment, and it is unusual because the experimental setup is easily managed by the users themselves. The beamline staff are excellent, and we are particularly grateful for their expertise in data processing, which was invaluable.” | |
 |
|
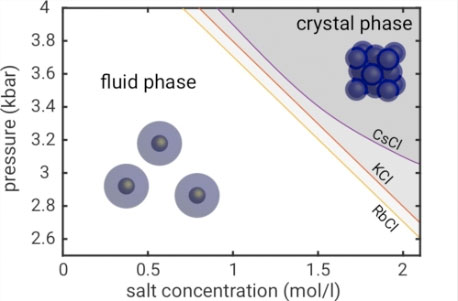
| Fig. 2: Pressure – salt concentration phase diagram of AuNP@PEG. For low pressures, the particles are in the liquid state, beyond a critical pressure, face-centred cubic (fcc) superlattices are formed within solution. The crystallisation transition depends on the salt concentration as well as on the salt type. | |
| The resulting pressure-salt concentration phase diagram shows that the crystallisation is a result of the combined effect of salt and pressure on the PEG coatings. Supercrystal formation occurs only at high salt concentrations, and is reversible. Increasing the salt concentration leads to a continuous decrease of the crystallisation pressure, whereas the lattice structure and degree of crystallinity is independent of the salt type and concentration. | |
| When reaching the crystallisation pressure, supercrystals form within the whole suspension; compressing the liquid further results in changes of the lattice constant, but no further crystallisation or structural transitions. This technique should be applicable to a variety of nanomaterials, and future studies may reveal insights into supercrystal formation that will help to understand crystallisation processes and enable the development of new and quicker methods for the synthesis of NP supercrystals. | |
| The NP crystallisation appears to be instantaneous, but in this set of experiments there was a delay of around 30 seconds between applying the pressure and taking the SAXS measurements. Dr Schroer and his team are returning to Diamond later this year to carry out time-resolved studies to further investigate this phenomenon. |
| Source: Diamond Light Source | |
|
Subscribe to a free copy of one of our daily Nanowerk Newsletter Email Digests with a compilation of all of the day's news. |
