| Posted: Sep 17, 2018 | |
Understanding surface science to manufacture quality cosmetics(Nanowerk News) A research team, affiliated with Ulsan National Institute of Science and Technology (UNIST) has examined the rates of liquid penetration on rough or patterned surfaces, especially those with pores or cavities. Their findings provide important insights into the development of everyday products, including cosmetics, paints, as well as industrial applications, like enhanced oil recovery. |
|
| This study has been jointly led by Professor Dong Woog Lee and his research team in the School of Energy and Chemical Engineering at UNIST and a research team in the University of California, Santa Barbara. Published online in the July 19th issue of the Proceedings of the National Academy of Sciences ("Rates of cavity filling by liquids"), the study identifies five variables that control the cavity-filling (wetting transition) rates, required for liquids to penetrate into the cavities. | |
 |
|
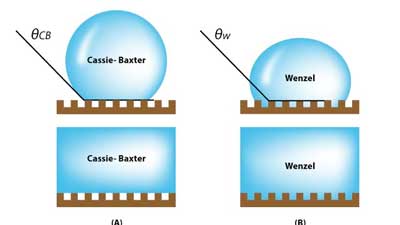
| This is the wetting transition from the Cassie-Baxter state to the Wenzel state on textured surfaces. (Image: UNIST) | |
| In the study, Professor Lee fabricated silicon wafers with cylindrical cavities of different geometries. After immersing them in bulk water, they observed the details of, and the rates associated with, water penetration into the cavities from the bulk, using bright-field and confocal fluorescence microscopy. Cylindrical cavities are like skin pores with narrow entrance and specious interior. The cavity filling generally progresses when bulk water is spread above a hydrophilic, reentrant cavity. | |
| As described in "Wetting Transition from the Cassie-Baxter State to Wenzel State", the liquid droplet that sits on top of the textured surface with trapped air underneath will be completely absorbed by the rough surface cavities. | |
| Their findings revealed that the cavity-filling rates are affected by the following variables: (i) the intrinsic contact angle, (ii) the concentration of dissolved air in the bulk water phase, (iii) the liquid volatility that determines the rate of capillary condensation inside the cavities, (iv) the types of surfactants, and (v) the cavity geometry. | |
| "Our results can used in the manufacture of special-purpose cosmetic products," says Professor Lee. "For instance, pore minimizing face primers and facial cleansers that remove sebum need to reduce the amount of | |
 |
|
| These are the micropatterned surfaces fabricated for this study. (Image: UNIST) (click on image to enlarge) | |
| On the other hand, beauty products, like sunscreens should be designed to protect the skin from harmful sun, while preventing pores clogging. Because, clogged pores hinder the skin's function of breathing or exchange of carbon dioxide and then cause further irritation, pimples, and blemished areas on your skin. In this case, it is better to reduce volatility and increase the amount of dissolved air in the cosmetic products, as opposed to facial cleansers. | |
| "This knowledge of how cavities under bulk water are filled and what variables control the rate of filling can provide insights into the engineering of temporarily or permanently superhydrophobic surfaces, and the designing and manufacturing of various products that are applied to rough, textured, or patterned surfaces," says Professor Lee. "Many of the fundamental insights gained can also be applied to other liquids (e.g., oils), contact angles, and cavities or pores of different dimensions or geometries." |
| Source: Ulsan National Institute of Science and Technology | |
|
Subscribe to a free copy of one of our daily Nanowerk Newsletter Email Digests with a compilation of all of the day's news. |
