| Posted: Sep 19, 2018 | |
Interfacial engineering core@shell nanoparticles for active and selective direct H2O2 generation(Nanowerk News) Hydrogen peroxide (H2O2) is a versatile chemical in modern industry, widely applied in many different fields. |
|
| To date, H2O2 is industrially manufactured by an indirect process that involves the sequential hydrogenation and oxidation of alkyl anthraquinone, which is however a multi-step process with high-cost and energy-intensive. | |
| On the sharp contrary, the direct synthesis of H2O2 from H2 and O2 is expected to be the most efficient way to produce H2O2 due to the remarkable advantages of atom economy, low energy consumption and only by-product of H2O. | |
| Hitherto, the direct synthetic route is mainly achieved by the supported Pd-based catalysts. The major problem associated with that is related to the low selectivity of H2O2. | |
| Despite great efforts have been devoted to constructing the Pd-based catalysts by introducing the second metals, understanding high-performance Pd-based catalysts for the direct H2O2 generation from either deep characterization or theoretical investigation are still extremely limited. | |
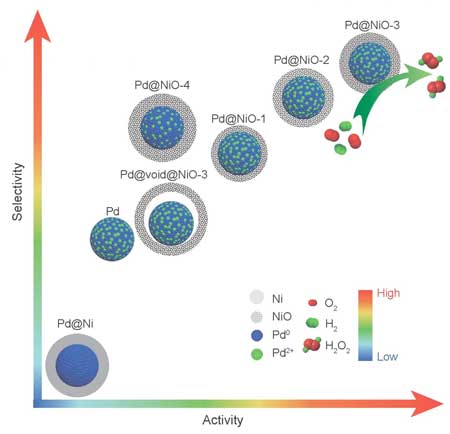 |
|
| This is a schematic illustration showing the activity and selectivity toward H2O2 synthesis of 5 wt% Pd@Ni-3/TiO2, 5 wt% Pd@NiO-x/TiO2 (x = 1, 2, 3, 4), 5 wt% Pd@void@Ni-3/TiO2 and 5 wt% Pd/TiO2. (Image: Science China Press) | |
| In a new overview published in the Beijing-based National Science Review ("Surface engineering at the interface of core/shell nanoparticles promotes hydrogen peroxide generation"), scientists at the Soochow University present the latest advances in direct H2O2 generation. | |
| Co-authors Yonggang Feng, Qi Shao, Bolong Huang, Junbo Zhang, and Xiaoqing Huang developed a class of Pd@NiO-x nanoparticles with unique core@shell interface structure, which achieves high activity, selectivity and stability for the direct H2O2 synthesis. | |
| These scientists likewise interpreted the mechanism from both electronic and energetic views. | |
| "Traditional Pd-based catalysts are very active for the side reactions, such as the decomposition and hydrogenation of H2O2 as well as the formation of H2O," they state in an article titled "Surface engineering in the interface of core/shell nanoparticles promotes hydrogen peroxide generation". | |
| "It is considered that the intrinsic surface property of Pd-based catalysts is essential for the selectivity and activity of the direct H2O2 synthesis," they add. "This arises because the barrier for O-O bond scission is sensitive to Pd surface structure, the key parameter governing H2O2 synthesis and decomposition activity." | |
| The creation of porous NiO shell is beneficial for exposing Pd active sites and thus enhancing the productivity of H2O2. | |
| "By tuning the composition of Pd@NiO-x NPs and the reaction condition, the efficiency of H2O2 synthesis could be well optimized with 5 wt% Pd@NiO-3/TiO2 exhibiting the highest productivity (89 mol/(kgcath)) and selectivity (91%) to H2O2 as well as excellent stability," they state. | |
| "The first principles simulations further revealed the mechanism from both electronic and energetic views," the scientists interpreted. "The superiority in selectivity is achieved by a spontaneous bond scission of H-H and charge transfer from O20 to O22- within the cavity of NiO interfacing with Pd surface.," | |
| "The high selectivity and activity making it one of the best catalysts for the direct H2O2 synthesis reported to date," they add. "The present work reported here highlights the importance of surface and interface engineering of Pd-based catalysts for the direct H2O2 synthesis with largely enhanced activity and selectivity." |
| Source: Science China Press | |
|
Subscribe to a free copy of one of our daily Nanowerk Newsletter Email Digests with a compilation of all of the day's news. |
