| Posted: Oct 10, 2018 | |
Synergy in two-dimensional materials and membranes research(Nanowerk News) Where researchers who worked with two-dimensional materials and those who worked with membranes were once separate, synergistic opportunities are resulting in exciting new developments, a Vanderbilt University chemical and biomolecular engineering professor has both opined and proven. |
|
| In a review published in Advanced Materials ("Facile Fabrication of Large-Area Atomically Thin Membranes by Direct Synthesis of Graphene with Nanoscale Porosity"), Assistant Professor of Chemical and Biomolecular Engineering Piran Kidambi and his team explored new interest in using materials only one atom thick for membrane applications. | |
| They explained the landscape on how the technology evolved and advanced and how the field is ripe for collaborations. Their technology road map suggested that research on two-dimensional materials and membranes were once separate fields, but synergistic opportunities are resulting in exciting new developments at their intersection. | |
 |
|
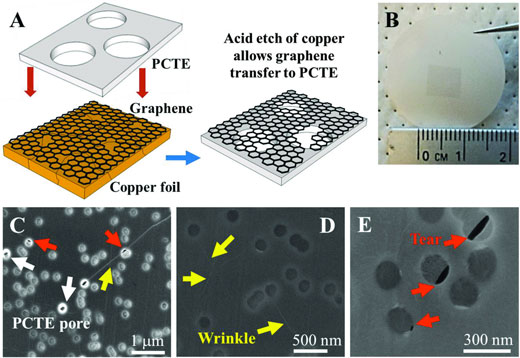
| Polymer-free graphene transfer to polycarbonate track etched (PCTE) supports. A) Schematic of graphene transfer process to polycarbonate track etched (PCTE) supports with 200 nm pores. Nanoporous graphene on copper foil is mechanically pressed against PCTE followed by etching of copper. B) Optical image of graphene on PCTE support. The black square in the image is graphene. C–E) Scanning electron microscopy (SEM) images of graphene on PCTE support. Red arrows indicate tears inevitably introduced during the mechanical pressing during transfer, yellow arrows show wrinkles in the graphene film and white arrows show open PCTE pores ∼200 nm without graphene. (© Wuiley) | |
| Kidambi and his team more recently applied that overlap in their own work to address some of the most critical challenges in membrane research: achieving high flow-through membranes without compromising filtration performance. | |
| The team initially focused on developing methods to directly form nanoscale holes into an atomically thin material. The team dialed down the temperature during graphene and found this resulted in nanoscale holes - missing carbon atoms from the two-dimensional layer of them bonded in a hexagonal lattice. | |
| "It reminded me of decreasing the temperature while baking a chocolate cake to get a different texture," Kidambi said. | |
| However, the atomically thin graphene with nanoscales holes needed to be supported to form a membrane. The team turned to conventional polymer membrane manufacturing techniques and decided to spread a thin polymer layer on the nanoporous graphene and dipped the stack into a water bath. | |
| The dip transformed the polymer to a porous support layer with graphene on the top, effectively forming an atomically thin membrane. | |
| "Continuing on with the baking analogy, this was like dough transforming into porous bread - the support polymer layer." | |
| The team used these atomically thin membranes to demonstrate separation of salt and small molecules from small proteins. | |
| "Most commercial membranes achieve separation at small size ranges by making a dense polymer layer that is several microns thick with tortuous pores," Kidambi said. "Diffusion across these layers is very slow. Here, we make membranes that are one atom thick and show much higher permeance - up to 100 times greater than the state-of-the-art commercial dialysis membranes - specifically in the low molecular weight cut-off range. | |
| "We think these membranes could offer transformative advances for small molecule separation, fine chemical purification, buffer exchange and a number of other processes including lab-scale dialysis." |
| Source: Vanderbilt University | |
|
Subscribe to a free copy of one of our daily Nanowerk Newsletter Email Digests with a compilation of all of the day's news. |
