| Dec 07, 2018 | |
Cryo-electron microscopy shows how stabilizing proteins bind to microtubules(Nanowerk News) By using three dimensional (3D) cryo-electron microscopy, RIKEN scientists have gleaned new insights into how stabilizing proteins bind to microtubules—the ‘conveyor belts’ of cells (Journal of Cell Biology, "Structural insight into microtubule stabilization and kinesin inhibition by Tau family MAPs"). These findings have implications for research into dementia and heart failure. |
|
| Microtubules both help maintain cell shape and form the basis of a large transportation network that transfers molecules within cells (Fig. 1). Tau-family microtubule-associated proteins (tau-MAPs) play important roles in stabilizing and aligning microtubules and thus in supporting their function. Tau-MAP malfunctions can lead to conditions such as neurodegenerative disorders and congestive heart failure. | |
 |
|
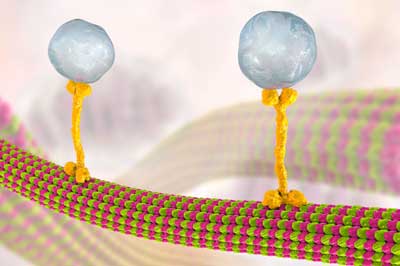
| Microtubules convey molecules between cells in vesicles (large blue-gray spheres), which are transported along a microtubule by a kinesin motor protein (yellow bone-shaped structure). Microtubules are made of dimers of the proteins alpha and beta tubulin (pink and green blobs). RIKEN researchers have found that microtubule-associated protein 4 can inhibit kinesin’s movement. (© Kateryna Kon) | |
| Despite much research on the functions of tau-MAPs, studying their detailed structures has been challenging because they continuously change shape. Now, cell biologist Ryo Nitta of the RIKEN Center for Biosystems Dynamics Research and colleagues from Kobe University and other institutions in Japan have overcome this problem and revealed how one tau-MAP, called MAP4, attaches to microtubules. | |
| The researchers stabilized MAP4 on a microtubule by using kinesin-1, a motor protein that transports cargo along microtubules. They then took many images of the complex from different angles using cryo-electron microscopy, where flash freezing fixes the sample in a close-to-physiological state. Finally, the team constructed a 3D representation of the complex from these images. | |
| The scientists found that one part of MAP4 strongly anchors to a groove between tubulin heterodimers consisting of alpha and beta tubulin, which bind together to form a microtubule. MAP4 binding in this way stabilizes the microtubule. | |
| Another part of MAP4 weakly binds to the surface of the tubulin dimer. But kinesin-1 also binds to this location, removing the part of MAP4 that was attached there. The now loose part of MAP4 folds and gathers above its anchor point on the microtubule, interfering with kinesin-1’s movement. | |
| “Tau-MAPs had been thought to compete with kinesin for the microtubule-binding site,” says Nitta. “But we found that MAP4 can bind to the microtubule at the same time as the kinesin motor protein, and we discovered ways in which MAP4 can inhibit kinesin’s function.” | |
| Malfunctions in tau-MAPs lead to disease. For example, when the protein tau acquires too many phosphate groups, it does not bind well to the microtubule, leading to degeneration of nerve fibers and dementia. Over-expression of MAP4 in heart muscle cells stabilizes microtubules too much, which can lead to thickening of the heart wall and congestive heart failure. | |
| “If we can control the stabilization of microtubules using tau-MAPs, we could control several diseases,” notes structural biologist Hideki Shigematsu of the RIKEN SPring-8 Center. | |
| Further research should focus on obtaining higher resolution images of MAP4’s structure and visualizing the MAP4-microtubule structure inside cells, says Shigematsu. |
| Source: RIKEN | |
|
Subscribe to a free copy of one of our daily Nanowerk Newsletter Email Digests with a compilation of all of the day's news. |
