| Dec 14, 2018 | |
Modification of nitrile latex with magnetite nanoparticles to prevent glove contamination(Nanowerk News) NBR (nitrile- butadiene rubber) gloves are used extensively in food and pharmaceutical industries when handling products. However, poor elasticity of NBR may cause small pieces of the glove to be torn away during manufacturing process and mixed with the products (known as "glove contamination”). |
|
| Regardless of its size, any type of material (e.g. rubber) contamination found in a finished food product can become a public relations nightmare for both the brand and the food manufacturer. A small piece of glove contamination can result in a withdrawal of the entire production run and potential lawsuits. Contamination accidents not only requires production line stoppages and incur huge costs, but also involve vast amount of time and money to regain consumer trust and rebuild the shattered brand image. | |
| Thus, effective measures to prevent food products’ contamination is a major concern for food manufacturers. | |
| Finding a solution to glove contamination issues has been a challenge for a long time since ordinary gloves cannot be detected by metal detectors. One of the solutions for the problem mentioned is the use of magnetic detectable gloves that can be made from various magnetic mineral materials such as iron oxide, or metals such as steel, lead, silver and chromium. | |
| Most of these gloves are made by incorporating a proportion of magnetic material. The size and concentration of magnetic material is intended to allow a homogeneous spread of the material throughout the glove to ensure that all parts of the glove are detectable by a detector. Thus, there is a need to provide improved magnetic gloves that has more sensitivity in detection while remain wearable by an end user. | |
 |
|
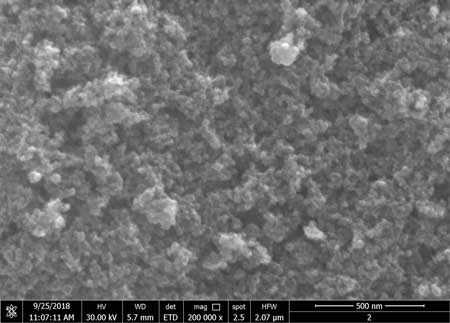
| SEM image of iron oxide. (Image: NANOCAT) | |
| Iron oxide nanoparticles possess unique features compared to equivalent larger-scale materials. Among iron oxide phases, such as magnetite (Fe3O4O), maghemite (γ-Fe2O3) and hematite (α-Fe2O3), magnetite is frequently used because of its high saturation magnetization value. Therefore, iron oxide magnetic nanoparticles have received significant interest for biomedical applications, mineral separation, magneto- optic materials and microwave filters. | |
| However, iron oxide nanoparticles have extremely high adhesion properties, resulting in the tendency for iron oxide nanoparticles to aggregate together. This is because iron oxide nanoparticles have a strong magnetic dipole-dipole interaction between particles in conjunction with a large surface energy. This problem limits the use of iron oxide nanoparticles. | |
| Thus, for industrial applications, it is quite important to develop techniques to control the dispersion or agglomeration phenomena of the iron oxide nanoparticles to be applied into functional materials and products. | |
| University of Malaya researchers came up with this present invention which relates to a method for producing an iron oxide nanoparticle that may be formed as a stable slurry (Patent WO 2017/065599 Al). | |
| A magnetically detectable glove comprised of at least one layer of the polymeric material that includes coated iron oxide nanoparticles. The new form of coated iron oxide nanoparticle has better magnetic properties and remains homogeneously dispersed in a liquid over an extended period. Nanoparticles and/ or the slurry have small particle size and excellent dispersion properties in a polymer matrix when a coating layer is formed on the said iron oxide nanoparticles. The nanoMAG slurry is measured using Vibrating Sample Magnetometer (VSM) for its magnetisation strength. Scanning Electron Microscopy (SEM) is used to measure the nanoMAG’s nanoparticle size as targeted. |
| Source: University of Malaya | |
|
Subscribe to a free copy of one of our daily Nanowerk Newsletter Email Digests with a compilation of all of the day's news. |
