| Jan 09, 2019 | |
Self-sorting through molecular geometries(Nanowerk News) Supramolecular assemblies are nanostructures resulting from molecules binding together, through intermolecular interactions, into larger units. One approach for controlling supramolecular assembly involves self-sorting: molecules recognizing copies of themselves, and binding with them. |
|
| Now, the findings (Communications Chemistry, "Ring shape-dependent self-sorting of pillar[n]arenes assembled on a surface") of an interdisciplinary collaboration between the Supramolecular group (Tomoki Ogoshi and coworkers) Atomic Force Microscopy (AFM) group (Hitoshi Asakawa, Takeshi Fukuma, and coworkers) of the Nano Life Science Institute (WPI-NanoLSI) Kanazawa University showed that self-sorting behavior can arise from the principle of geometrical complementarity by shape: in a mixture of specific pentagonal and hexagonal molecular building blocks, pentagons bind to pentagons and hexagons to hexagons, and no mixing occurs. | |
 |
|
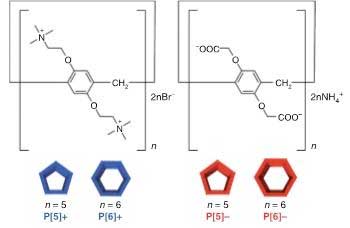
| Structures of pillar[n]arenes, n = 5 and 6. Left, blue: cationic (positively charged) variants; right, red: anionic (negatively charged) variants. (Image: Kanazawa University) | |
| Asakawa and members of the AFM group conducted experiments with molecules called pillar[n]arenes, with n = 5 and n = 6, corresponding to pentagonal and hexagonal shapes, respectively. Both molecules come in two 'flavors': positively (cationic) or negatively charged (anionic). The polygonal molecules are essentially rings of 5 or 6 identical organic units, each featuring a benzene ring, but the composition of the units is different for the cationic and the anionic variants. | |
| Ogoshi and his colleagues of the Supramolecular group let cationic pillar[5]arenes (P[5]+ in shorthand notation) adsorb on a quartz substrate. From this structure, they were able to grow P[5]+/P[5]-/P[5]+/... multilayers by immersing it alternatingly in anionic and cationic pillar[5]arene solutions. | |
| The addition of a layer was verified each time by ultraviolet-visible spectroscopy measurements. The resulting overall structure is a 'nanomat' of tubular structures with pentagonal pores. Similar results were obtained for the pillar[6]arenes: stacks of alternating cationic and anionic layers of the hexagonal molecules could be easily fabricated. The arrangement of pillar[n]arenes on a surface was investigated by collaboration with Prof. Takanori Fukushima, Prof. Tomofumi Tada and co-workers from Tokyo Institute of Technology. | |
| What the scientists found surprising was that it was not possible to stack pentagonal and hexagonal building blocks when trying to build an anionic layer on a cationic one (and vice versa). This is a manifestation of self-sorting: only like polygons can self-assemble, even if ionic interactions drive the formation of cation-anion layered structures. | |
| The researchers also examined the structure of the first layer of P[5]+ or P[6]+ molecules on the quartz substrate. For the hexagonal molecules, the two-dimensional packing structure did no exhibit long-range structural order, whereas for the pentagonal molecules, it did. This is partly attributed to a lower density for the latter. For the multilayer 'nanomats', the same trend was observed: long-range order for the pentagonal stacks. The ring shape-dependent packing structures were simulated by a Monte Carlo simulation by collaboration with Prof. Tomonori Dotera from Kindai University. | |
| The self-sorting effect discovered by Ogoshi and colleagues has promising potential applications. Quoting the scientists: "The ultimate challenge will be to propagate cavity-shape information on the surface to provide shape-recognisable adsorption and adhesive materials." |
| Source: Kanazawa University | |
|
Subscribe to a free copy of one of our daily Nanowerk Newsletter Email Digests with a compilation of all of the day's news. |
