| Feb 13, 2019 | |
Physicists watch electron transfer in a single molecule(Nanowerk News) The building blocks of matter surrounding us are atoms and molecules. The properties of that matter, however, are often not set by these building blocks directly, but rather by their mutual interactions, which are governed by their outer electron shells. Many chemical processes are based on the exchange of electrons between atoms and molecules, a mechanism known as electron transfer. |
|
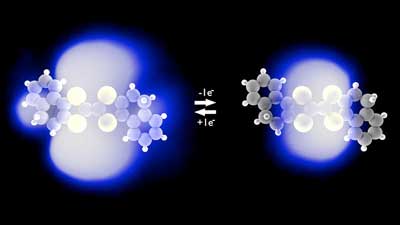 |
|
| Change in the electron cloud of a single molecule upon charging. (Image: Laerte Patera & Jascha Repp) | |
| Atoms and molecules are so unimaginably small that a direct imaging of these basic building blocks and their interaction seemed, for a long time, impossible. | |
| Several decades ago, imaging individual atoms became possible thanks to the invention of sophisticated types of microscopies that are not based on optics. Instead, the objects are sensed with an atomically sharp tip that can image matter down to the ångström scale, where 10 million ångström equal 1 millimeter in space. | |
| Among these microscopies, the so called scanning tunneling microscopy images the electron shell of matter by current measurement. With this type of microscopy, it is possible to probe the electron clouds surrounding atoms and molecules, known as electron orbitals. | |
| Another variant of these microscopies, the atomic force microscopy is instead, by sensing tiny forces, able to resolve individual bonds between neighboring atoms. Since roughly one decade, stunning images of a single molecule’s chemical structure may be acquired with this technique. | |
| While electron orbitals are decisive for virtually all chemical reactions, in reverse, chemical reactions also lead to dramatic changes of the shape of orbitals. However, this back-action to the electron shell, when atoms and molecules exchange charges with their neighbors, could not be imaged so far. | |
| As scanning tunneling microscopy is based upon the measurement of currents, it requires a conductive support; yet this support allows only one single stable charge state for a molecule. In fact, any additional charge tends to escape into the underlying support, hindering the microscopic observation of the effect of electron transfer on the molecular orbitals. | |
| Therefore, molecules need to be examined on an electrically insulating support, should one wish to study different charge states. This is, in principle, possible through atomic force microscopy, but this type of microscopy cannot be used to measure the outer electron shell. | |
| At the University of Regensburg, the above back-action of the electron transfer onto the electron orbitals has now, for the first time, been captured by images (Nature, "Mapping orbital changes upon electron transfer with tunnelling microscopy on insulators"). | |
| The international team of scientists accomplished this breakthrough by combining principles of the two aforementioned scanning tunneling and atomic force microscopy, thereby developing a novel variant. | |
| Instead of the usual direct current in conventional scanning tunneling microscopy, in their experiments they drive a tiny alternating current between the atomically sharp conductive sensor and the molecule under study. | |
| The alternating current consists of just a single electron that is driven to jump back and forth between the sensor’s tip and the molecule. This way, no net current flows inside the microscope, which therefore does not require a conductive support of the molecule. | |
| This in turn allows one to bring the molecule in any desired charge state, that is, it allows forcing the molecule to give away or take up additional electrons as it would in a chemical reaction, while studying its orbitals more closely. | |
| With this novel method, the researchers could, for the first time, record images of the changes of the electron shell that occur upon the charging of molecules, directly resolved in space at the single-molecule level. | |
| These novel microscopic insights into the atomistic details of electron transfer on the single-orbital scale will shed new light on our understanding of processes at the heart of vital chemical reactions such as photosynthesis, combustion and corrosion. |
| Source: Universität Regensburg | |
|
Subscribe to a free copy of one of our daily Nanowerk Newsletter Email Digests with a compilation of all of the day's news. |
