| Mar 18, 2019 | |
A ground-breaking chemical protocol: on-surface synthesis of acene polymers(Nanowerk News) The optical and electronic properties of π-conjugated polymers have placed them in the paradigm for organic electronics. Despite the great advances in the field, the poor solubility of many of these compounds precludes their synthesis by standard chemistry methods, such is the wet chemistry. |
|
| For example, acene compounds (fused benzene rings in a rectilinear arrangement) present a great potential for plastic electronics, but the design of high-quality conjugated polymers based on acene building units has not been fully achieved to date. Herein, on-surface synthesis has become a powerful tool to design with atomistic precision a variety of nanomaterials. | |
| In the recent publication in Angewandte Chemie Int. Ed. ("On-Surface Synthesis of Ethynylene-Bridged Anthracene Polymers"), researchers led by professors David Écija, Nazario Martín, Pavel Jelínek and Jonas Björk have reported an extensive study of the on-surface synthesis of poly(p-anthracene ethynylene) molecular nanowires on a gold surface, using scanning tunneling microscopy (STM), atomic force microscopy (AFM) and density functional theory (DFT). | |
 |
|
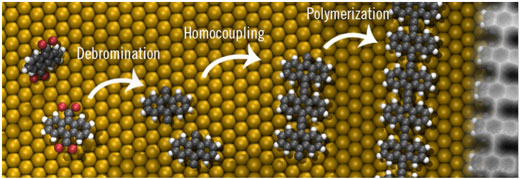
| On-surface synthesis of ethynylene bridged anthracene polymers on pristine gold. | |
| The novel chemical approach is based on the dehalogenation, homocoupling and aromatization of a quinoid anthracene precursor endowed with =CBr2 moieties. Annealing to 400K enabled the debromination and, after diffusion of the species, long molecular wires up to 30 nm without defects were formed. The scanning probe microscopy revealed the spatial distribution of the valence and conductance edges, resulting in a low band gap of 1.5 eV, confirmed by the DFT calculations. | |
| Nobody up to date succeeded in polymerizing the acene family. This challenge was overcome by combining approaches from organic chemistry (Prof. Nazario Martín) and on-surface chemistry (Prof. David Écija). In the synthesis, suitable precursor species were equipped with appropriate functional groups to steer the formation of ethynylene bridges (-C☰C-). This elegant coupling reaction has few side-products, and allows the detailed atomistic study of the molecular structure. | |
| Notably, its low band gap sets the structure as an interesting nanomaterial for optoelectronic devices. Further steps will be necessary and are in progress to study the optimal transference of the polymers onto functional devices. | |
| Écija remarks that “The study is exhaustive; includes not only the synthesis of a novel branch of polymers, we also elucidated its electronic properties along the macromolecule”. The authors are confident in the new possibilities arising: “We believe this breakthrough will bring new possibilities for the development of plastic electronic devices, and now there is much work ahead,” Santos, coauthor of the open access publication, says. | |
| For example, the transference of porous graphene to other substrates has been recently demonstrated which opens new avenues to bring these new nanomaterials to promising applications. | |
| This work is a multidisciplinary collaboration between scientists from IMDEA Nanociencia (Madrid), the Complutense University of Madrid, The Regional Centre of Advanced Technologies and Materials (Czech Republic), The Czech Academy of Sciences, and the Linköping University. |
| Source: IMDEA Nanociencia | |
|
Subscribe to a free copy of one of our daily Nanowerk Newsletter Email Digests with a compilation of all of the day's news. |
