| Mar 19, 2019 | |
Cost-effective nanoparticle method for hydrogen fuel production process(Nanowerk News) Nanoparticles composed of nickel and iron have been found to be more effective and efficient than other, more costly materials when used as catalysts in the production of hydrogen fuel through water electrolysis (Nanoscale, "Controlling the 3-D morphology of Ni–Fe-based nanocatalysts for the oxygen evolution reaction"). |
|
| The discovery was made by University of Arkansas researchers Jingyi Chen, associate professor of physical chemistry, and Lauren Greenlee, assistant professor of chemical engineering, as well as colleagues from Brookhaven National Lab and Argonne National Lab. | |
 |
|
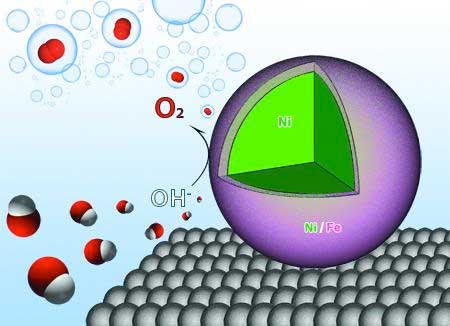
| Researchers at the U of A have designed nanoparticles that act as catalysts, making the process of water electrolysis more efficient. (Image: Jingyi Chen, Lauren Greenlee and Ryan Manso) | |
| The researchers demonstrated that using nanocatalysts composed of nickel and iron increases the efficiency of water electrolysis, the process of breaking water atoms apart to produce hydrogen and oxygen and combining them with electrons to create hydrogen gas. | |
| Chen and her colleagues discovered that when nanoparticles composed of an iron and nickel shell around a nickel core are applied to the process, they interact with the hydrogen and oxygen atoms to weaken the bonds, increasing the efficiency of the reaction by allowing the generation of oxygen more easily. Nickel and iron are also less expensive than other catalysts, which are made from scarce materials. | |
| This marks a step toward making water electrolysis a more practical and affordable method for producing hydrogen fuel. Current methods of water electrolysis are too energy-intensive to be effective. |
| Source: University of Arkansas | |
|
Subscribe to a free copy of one of our daily Nanowerk Newsletter Email Digests with a compilation of all of the day's news. |
