| Aug 16, 2019 | |
How synthetic cells are fighting infectious bacteria(Nanowerk News) A team of researchers at the DWI - Leibniz Institute for Interactive Materials in Aachen is pioneering a revolutionary concept for antimicrobials. In their latest work published in Nano Letters ("Membrane-Mimetic Dendrimersomes Engulf Living Bacteria via Endocytosis") in collaboration with the University of Pennsylvania, Temple University, RWTH Aachen University, and the Max Planck Institute for Medical Research, they developed artificial cells that can detect and devour bacteria. |
|
| These artificial phagocytic cells present an alternative to antibiotic drugs, that are the mainstream treatment against bacterial infections. However, some bacteria have learned to become resistant and even thrive in the presence of antibiotics. Already now, worldwide 700,000 people die each year because of bacteria resistant to antibiotics and this figure is estimated to skyrocket to 10 million by 2050. | |
| “Antibiotic and other antimicrobials are designed to target specific structures in bacteria. Yet, these smart bugs develop strategies to become immune to them. Our approach is radically different. Our artificial cell eats up the whole bacterium in a single bite not giving them the chance to develop resistance,” Rodriguez-Emmenegger, the leading author of this research, says. | |
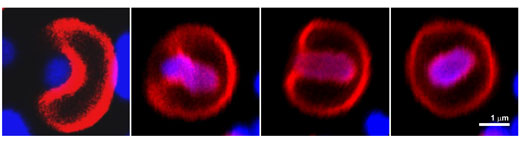
| The first step is the binding of a bacterium to the membrane of the synthetic cell. This process causes the membrane to wrap and engulf the bacterium inside of a compartment resembling the digestive sack of macrophages. Once engulfed by the synthetic cell, the bacterium can no longer proliferate or form biofilms, robust slime layers embedding the bacteria. Now the isolated bacterium can be killed inside the synthetic cell keeping all the toxic debris away from the surrounding tissues. | |
| “We were so excited the first time we observed under the microscope that our concept really works,” Kostina, an author of this work says. “Of course this requires unique properties of the membrane of the synthetic cells,” Rahimi, a coauthor of the work explains. “We need that the membrane is sticky to bacteria and extremely flexible to allow wrapping. But at the same time it must remain stable in the biological milieu.” | |
| The combination of these properties has been the major bottleneck for artificial cells based on polymers or lipids. To overcome this obstacle, the authors utilized a new class of artificial cell membranes in which lipids or polymers were replaced by molecules called Janus dendrimers. | |
| Like Janus, the Roman god of duality, these molecules have water-loving and water-hating parts that drive the spontaneous assembly into artificial cells mimicking key properties of the membrane of their natural counterparts. | |
 |
|
| Microscopic images showing how synthetic cells (red) eat up a living bacterium (blue). (© Nano Letters) (click on image to enlarge) | |
| “Perhaps the most exciting feature of these artificial cells is that we can program in the molecular structure of the Janus dendrimers the flexibility and stability of the membrane as well as their ability to detect and selectively capture bacteria,” Kostina explains. “Now we have a very powerful tool to tailor the interactions with bacteria with exquisite precision”. | |
| Currently the team works on integrating natural receptors into the synthetic cell to endow them with the ability to tell apart between different strains of bacteria (PNAS, "Encoding biological recognition in a bicomponent cell-membrane mimic"). The authors believe that by introducing certain natural glycosphingolipids or proteins naturally present in cells their synthetic cells will be able to selectively capture pathogens while ignoring other bacteria. | |
| Rodriguez-Emmenegger together with Möller and Spatz –also co-authors of this work- are currently working in the translation of this research to minimize surgical replacement of orthopedic implants caused by bacterial colonization. By localizing these artificial cells on the surface of implants, they could act as an active scavenger, that only after sensing the presence of bacteria, isolates, and kill them in their interior without releasing any toxic molecules. This can ultimately prolong the life time of the implants and save enormous amount of suffering and cost to the patient.” | |
| Rodriguez-Emmenegger is convinced that this concept will set a new paradigm in the design and fabrication of smart antimicrobials by introducing a biomimetic mechanism that does not provide a selective pressure leading to the emergence of resistant bacterial strains. |
| Source: Leibniz Institute for Interactive Materials | |
|
Subscribe to a free copy of one of our daily Nanowerk Newsletter Email Digests with a compilation of all of the day's news. |
