| Jan 20, 2020 | |
Addressing global warming with new nanoparticles and sunshine(Nanowerk News) Harvesting sunlight, researchers of the Center for Integrated Nanostructure Physics, within the Institute for Basic Science (IBS, South Korea) published in Materials Today ("Phase-Selective Highly Efficient Nanostructured Metal-decorated Hybrid Semiconductors for Solar Conversion of CO2 to Absolute CO Selectivity") a new strategy to transform carbon dioxide (CO2) into oxygen (O2) and pure carbon monoxide (CO) without side-products in water. This artificial photosynthesis method could bring new solutions to environmental pollution and global warming. |
|
| While, in green plants, photosynthesis fixes CO2 into sugars, the artificial photosynthesis reported in this study can convert CO2 into oxygen and pure CO as output. The latter can then be employed for a broad range of applications in electronics, semiconductor, pharmaceutical, and chemical industries. | |
| The key is to find the right high-performance photocatalyst to help the photosynthesis take place by absorbing light, convert CO2, and ensuring an efficient flow of electrons, which is essential for the entire system. | |
| Titanium oxide (TiO2) is a well-known photocatalyst. It has already attracted significant attention in the fields of solar energy conversion and environmental protection due to its high reactivity, low toxicity, chemical stability, and low cost. | |
| While conventional TiO2 can absorb only UV light, the IBS research team reported previously two different types of blue-colored TiO2 (or “blue titania”) nanoparticles that could absorb visible light thanks to a reduced bandgap of about 2.7 eV. | |
| They were made of ordered anatase/disordered rutile (Ao/Rd) TiO2 (called, HYL’s blue TiO2-I) ("An order/disorder/water junction system for highly efficient co-catalyst-free photocatalytic hydrogen generation"), and disordered anatase/ordered rutile (Ao/Rd) TiO2 (called, HYL’s blue TiO2-II) ("Visible-Light-Driven, Metal-Free CO2 Reduction"), where anatase and rutile refer to two crystalline forms of TiO2 and the introduction of irregularities (disorder) in the crystal enhances the absorption of visible and infrared light. | |
 |
|
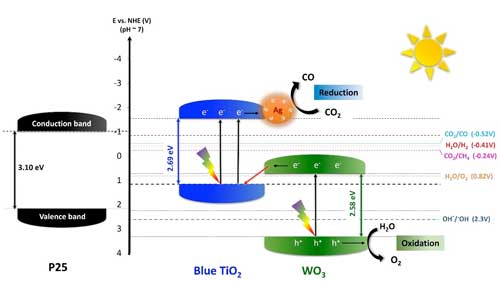
| Figure 1. Proposed energy diagram representing the electron transfer mechanism in TiO2/WO3-Ag hybrid nanoparticles. This so-called Z-scheme shows the flow of charged particles (electrons, e- and holes, ,h+) through the different components of the nanoparticles. Blue TiO2 and WO3’s e- can occupy lower (valence band, VB) and higher (conductive band, CB) energy levels. Photons from sunlight (thunders) provide the energy for the e- to jump up from the VB to the CB (black arrows pointing upwards), leaving h+ behind. TiO2’s lower band is close, just a bit lower than WO3’s higher band level, so e- from the high band of WO3 can migrate to the VB of blue TiO2 to trap its holes. After separation, the excited e- jump from the CB of TiO2 onto silver nanoparticles allowing the conversion of CO2 into CO, while the photogenerated h+ in the WO3 site oxidize water (H2O) to form oxygen (O2). (click on image to enlarge) | |
| For the efficient artificial photosynthesis for the conversion of CO2 into oxygen and pure CO, IBS researchers aimed to improve the performance of these nanoparticles by combining blue (Ao/Rd) TiO2 with other semiconductors and metals that can enhance water oxidation to oxygen, in parallel to CO2 reduction into CO only. | |
| The research team obtained the best results with hybrid nanoparticles made of blue titania, tungsten trioxide (WO3), and 1% silver (TiO2/WO3–Ag). | |
| WO3 was chosen because of the low valence band position with its narrow bandgap of 2.6 eV, high stability, and low cost. Silver was added because it enhances visible light absorption, by creating a collective oscillation of free electrons excited by light, and also gives high CO selectivity. | |
| The hybrid nanoparticles showed about 200 times higher performance than nanoparticles made of TiO2 alone and TiO2/WO3 without silver. | |
 |
|
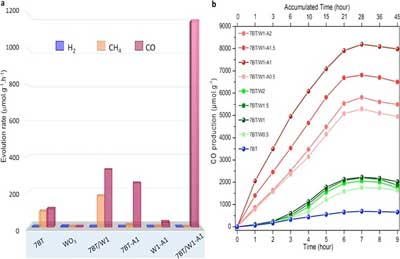
| Figure 2. Efficient and selective production of CO with different nanoparticles. (a) The graph shows that hybrid TiO2/WO3-Ag (7BT/W1-A1) nanoparticles are the best at selectively producing pure CO, without H2 and CH4 side products within a 7-hour timeframe. These can be compared with nanoparticles made of blue TiO2, WO3, hybrid TiO2/WO3 (7BT/W1) and hybrid TiO2/Ag (W1-A1). (b) CO production using different hybrid nanoparticles made of TiO2/WO3-Ag (red lines), TiO2/WO3 (green lines) and TiO2-only nanoparticles (blue lines) within 9 hours. 7BT/W1-A1 with a concentration of 1% silver has the best performance. (click on image to enlarge) | |
| Starting from water and CO2, this novel hybrid catalyst produced O2 and pure CO, without any side products, such as hydrogen gas (H2) and metane (CH4). The apparent quantum yield that is the ratio of several reacted electrons to the number of incident photons was 34.8 %, and the rate of reacted electrons 2333.44 µmol g-1h-1. The same measurement was lower for nanoparticles without silver (2053.2 µmol g-1h-1), and for nanoparticles with only blue TiO2 (912.4 µmol g-1h-1). |
| Source: Institute for Basic Science | |
|
Subscribe to a free copy of one of our daily Nanowerk Newsletter Email Digests with a compilation of all of the day's news. |
