| Aug 28, 2020 | |
Chelation - a new bonding mode for stable perovskite surface(Nanowerk News) Recent progresses in solution processed perovskite film have identified the detrimental roles of surface and grain boundaries, which usually caused structural degradation and trap-assisted recombination of solar cells. Toward these issues, surface engineering has been recognized as a basic strategy to gain high-performance perovskite solar cells. |
|
| Many organic molecules and polymers have been developed for surface passivation of perovskites. The earliest ones are fullerene derivative (e.g. PCBM) and Lewis base (e.g. pyridine, thiophene), which weakly interact with perovskite surface by Van der Waal or coordination bonds. Later, ionic molecules were anchored to perovskite, which fill the charged defect sites via relatively large electrostatic interactions. | |
| However, it is still challenging to get even stronger interaction strength to perovskite surface that can retain more reliable passivation under various environments. | |
| Yang and Hou’s group at East China University of Science and Technology developed a new type of bonding, i.e., chelation, by using diethyldithiocarbamate (DDTC) molecule as a chelating agent for surface engineering to the CsPbI2Br perovskite (see Fig.1). | |
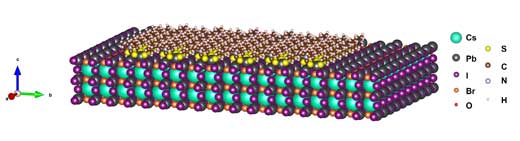 |
|
| Figure 1. Chelation of CsPbI2Br perovskite by diethyldithiocarbamate. (Image provided by the researchers) (click on image to enlarge) | |
| The team reported their findings in Nature Communications ("Surface chelation of cesium halide perovskite by dithiocarbamate for efficient and stable solar cells") | |
| The dithiocarbamate molecules can be coordinated to surface Pb sites via strong bidentate chelating bonding and form the lead diethyldithiocarbamate (Pb(DDTC)2) molecules. | |
| As proved by density functional theory (DFT), the Pb(DDTC)2 has a large adsorption energy of -1.73 eV on the surface, which is about 4-5 times larger than that of prevalent passivation molecules. | |
| Such strong chemical bonds between the surface under-coordinated defect and chelating agent enables a reliable passivated perovskite surface with long term stability under various environmental stimuli. | |
| Solar cell based on this chelated CsPbI2Br perovskite reaches a champion power conversion efficiency of 17.03%, a large open-circuit voltage of 1.37 V, and most importantly, an excellent device stability that can maintain 98% of its initial efficiency for over 1400 hours in ambient condition. | |
| These findings provide scientific insights on the surface engineering of perovskite that can facilitate the further development and application of perovskite optoelectronics. |
| Source: East China University of Science and Technology | |
|
Subscribe to a free copy of one of our daily Nanowerk Newsletter Email Digests with a compilation of all of the day's news. |
