| Dec 02, 2021 |
Strategies for reducing platinum waste in fuel cells
(Nanowerk News) Industry and university researchers used the Advanced Light Source (ALS) to explore why the platinum used as a catalyst in hydrogen fuel cells degrades unevenly (Advanced Energy Materials, "Probing Heterogeneous Degradation of Catalyst in PEM Fuel Cells under Realistic Automotive Conditions with Multi-Modal Techniques").
|
|
The resulting knowledge has enabled the development of simple, effective strategies to reduce the waste of precious catalyst material, lowering the costs associated with a promising green technology.
|
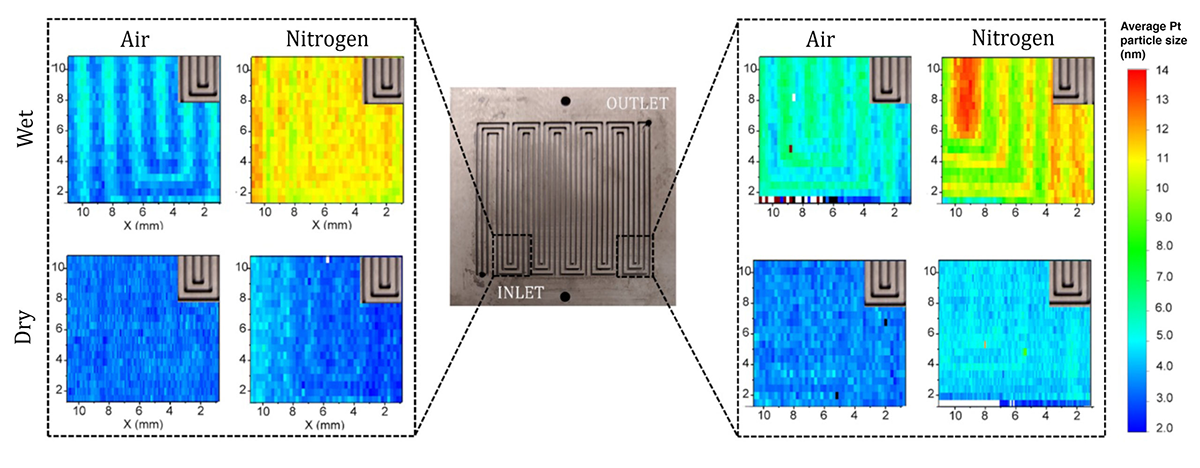 |
| X-ray microdiffraction revealed how various conditions affect catalyst particle size in flow fields—i.e., the channels that facilitate interaction between hydrogen gas and catalyst material in fuel cells. The components were subjected to accelerated stress testing that simulated tens of thousands of use cycles. Larger particle sizes (redder colors) indicate greater degradation. The x-ray diffraction maps are 1 cm x 1 cm. (click on image to enlarge)
|
Waste not, want not
|
|
Fuel cells are electrochemical devices that generate electricity from hydrogen—a “clean” fuel that produces only water when burned. For automotive applications, polymer electrolyte fuel cells (PEFCs) are attractive because they are powerful yet compact, and can quickly ramp up power output as needed. To achieve the required power, hundreds of fuel cells are combined in parallel to form a fuel cell “stack.”
|
|
Improving the durability of PEFC stacks and reducing their cost remain challenging, however. According to one Department of Energy breakdown for fuel cells in light-duty vehicles, precious-metal electrocatalysts account for 31% of stack cost. These precious metals are mostly platinum or platinum alloys that catalyze the required but sluggish oxygen reduction reaction on the PEFC cathode.
|
|
Relatively large quantities of platinum are often used to maintain sufficient performance, since active catalyst surface area is lost as a result of degradation over time. If the degradation is not spatially uniform, some still-active platinum can be left underutilized. To improve fuel cell durability and reduce waste, the detailed causes of uneven platinum degradation need to be better understood.
|
|
Scientists from the Bosch Research and Technology Center North America (Sunnyvale) and from UC Irvine’s National Fuel Cell Research Center joined forces to tackle the challenge of understanding how fuel cell durability is affected by variables such as relative humidity, type of feed gas, and geometry of the flow field (i.e., the maze-like channels that guide hydrogen gas through the catalyst layer).
|
|
The team studied commercially available catalyst-coated membranes that were subjected to accelerated stress testing (cycling between two voltages thousands of times to simulate aging) under wet and dry conditions in an air or nitrogen environment. The “postmortem” analysis of the samples involved a wide variety of techniques, including scanning electron microscopy, energy dispersive x-ray spectroscopy, x-ray fluorescence, and x-ray photoelectron spectroscopy.
|
|
At the ALS, the researchers used x-ray microdiffraction at Beamline 12.3.2 to quickly and efficiently map the sizes of the aged platinum nanoparticles (larger particles indicate greater degradation). They also used x-ray computed tomography at Beamline 8.3.2 to visualize catalyst layers and changes in their morphology after aging.
|
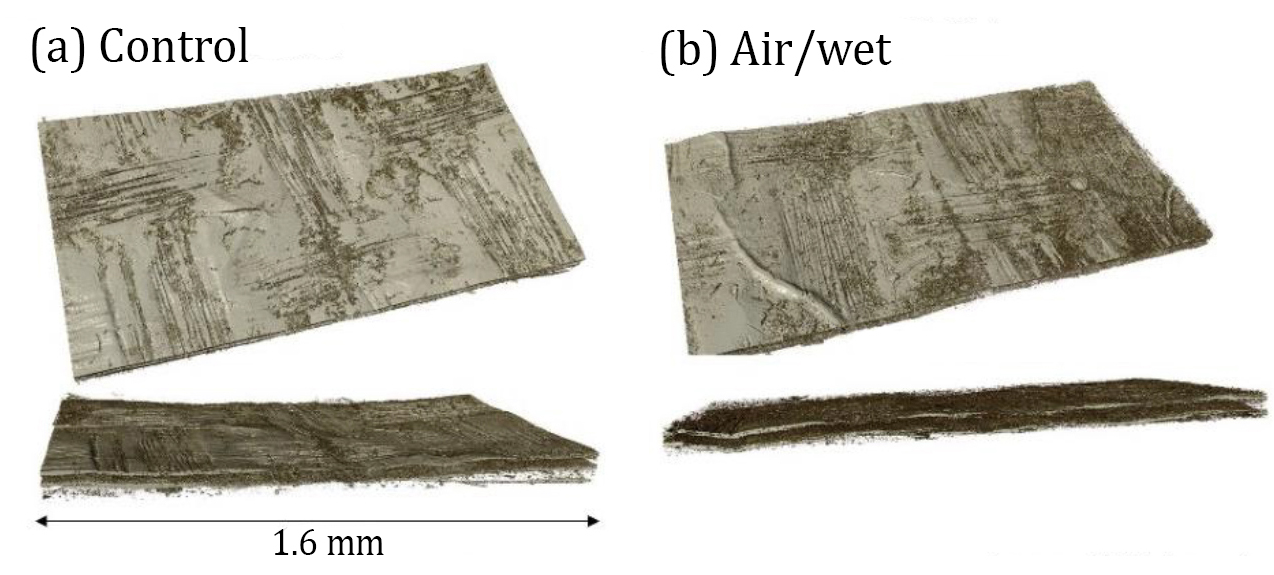 |
| X-ray computed tomography of membrane electrode assemblies before and after accelerated stress testing (control and air/wet conditions, respectively). The catalyst layer thickness remained approximately unchanged, with inhomogeneity in layer thickness mainly due to a woven texture.
|
Mitigation strategies identified
|
|
The results revealed that platinum nanoparticle size increased more near the flow-field inlet vs the outlet and in nitrogen environments with higher humidity. Additionally, nanoparticle size increased more in the “land” regions of the flow field (i.e., the spaces between channels) vs in the channels themselves. This land/channel effect disappeared for a flow-field design with narrower dimensions, and the addition of counter-flow channels could help reduce inlet/outlet variability.
|
|
Overall, it’s desirable to operate at sub-humidified conditions. Higher-temperature operation can help maintain lower relative humidity in the stack, but at the same time, it may contribute to degradation of other (non-catalyst) components. Therefore, careful optimization is needed.
|
|
PEFC vehicles are an excellent emission-free alternative to fossil-fuel vehicles. But PEFC cost and durability are barriers still to be overcome. The results from this study allow scientists to develop immediate system-level mitigation strategies to improve durability and reduce cost until fundamental material properties improve.
|


