| Jul 30, 2022 |
Hybrid semiconductors perform under pressure
(Nanowerk News) Supported by high-pressure studies at the Advanced Light Source (ALS), researchers found that compressing hybrid (organic–inorganic) semiconductors significantly boosts their conductivity (Angewandte Chemie International Edition, "Charge Reservoirs in an Expanded Halide Perovskite Analog: Enhancing High-Pressure Conductivity through Redox-Active Molecules").
|
|
The work demonstrates a novel doping mechanism in which the material’s organic molecules serve as charge reservoirs for tuning charge-carrier concentration, with promising applications in solar cells, lasers, and LEDs.
|
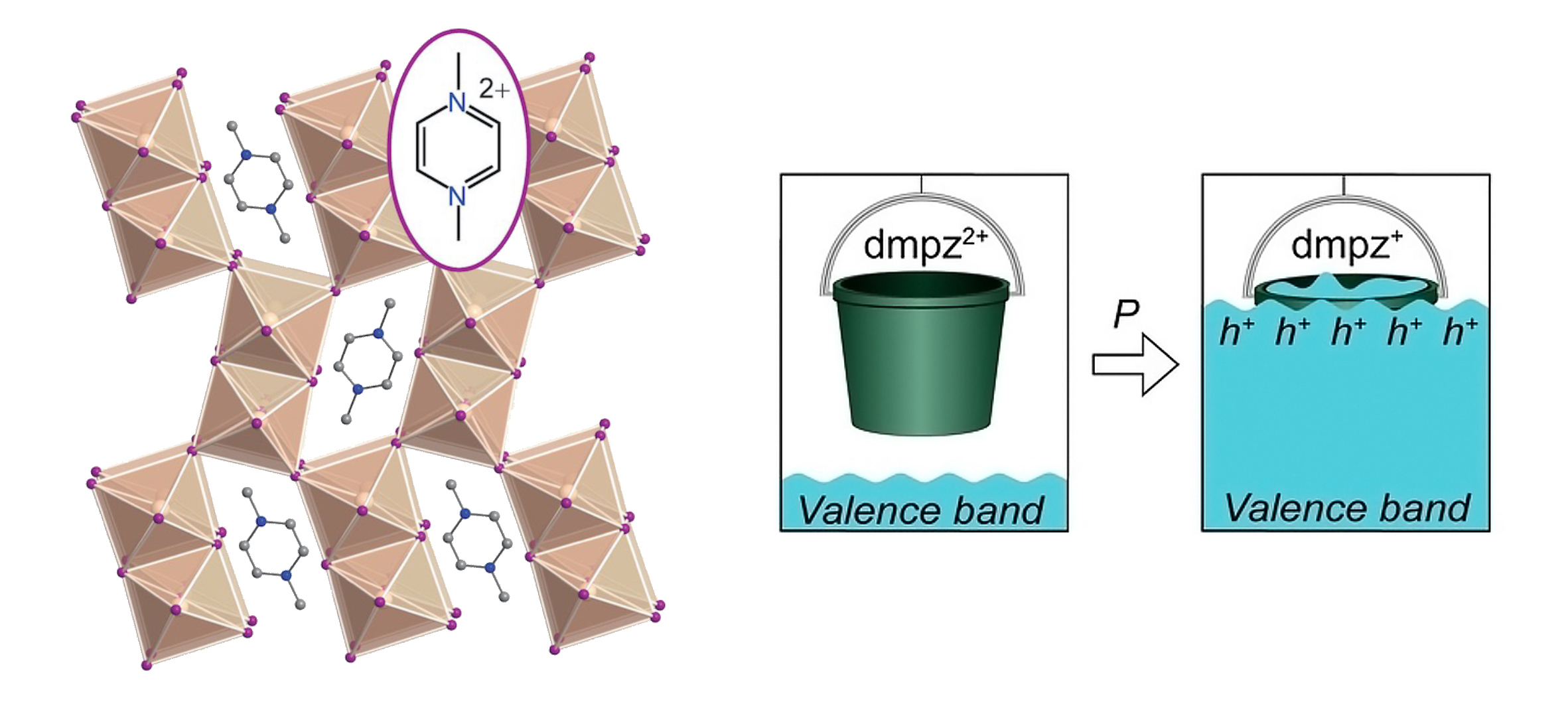 |
| Materials with cage-like octahedral structures (perovskites) have highly tunable electronic properties. The crystal structure depicted on the left is a of hybrid (organic–inorganic) perovskite-like analog. The inorganic component—pairs of edge-sharing tin iodide octahedra—form a framework able to fit a relatively large organic molecule—N,N′-dimethylpyrazinium (dmpz)—in its interstitial cavities. Compressing the material raises the level of the framework’s valence band (bucket analogy, right) to lie closer to the dmpz empty orbitals, which can then accept electrons from the framework. (Image: ALS)
|
Halide perovskite solutions
|
|
Tunable semiconductor materials containing organic compounds can be synthesized from solution using scalable, inexpensive methods. The resulting hybrid (organic–inorganic) compounds belong to a class of materials known as perovskites, named after the mineral in which their distinctive octahedral structures were first identified. The structures are stable, yet flexible enough to allow for variations to produce desired material properties.
|
|
In halide perovskites, metal ions (e.g., lead or tin) are enclosed in octahedral cages defined by halide ions (e.g., bromide or iodide). Such materials offer useful optoelectronic properties, including tunable band gaps and strong optical absorption. Accordingly, they are of great interest for use in applications such as light-emitting diodes (LEDs), lasers, and solar cells. In the latter case, lead iodide in particular has shown remarkable improvements in photovoltaic efficiency.
|
|
However, there are still fundamental challenges in need of solutions. For example, a greater understanding of how to control electronic transport properties in these materials via doping is needed to improve their performance as semiconductors and open up new possibilities.
|
Elbow room for doping
|
|
As with other semiconductors, doping in halide perovskites has mainly relied on the introduction of defects and impurities. However, these strategies are hindered by the formation of compensating defects in these self-assembling materials. To avoid this, a team led by researchers from Stanford University sought to incorporate redox-active (electron-accepting or -donating) molecules into the organic component of the perovskite.
|
|
Previously, the group had successfully synthesized a series of expanded halide perovskites, with cavities large enough to accommodate organic molecules with six-membered aromatic rings. In this work, the researchers synthesized an expanded perovskite analog using tin iodide for the inorganic framework and N,N′-dimethylpyrazinium (dmpz) as organic electron acceptors.
|
|
The band gap of this material is set by the energy difference between the empty low-lying orbitals of the dmpz and the filled orbitals of the tin iodide framework. However, the lowest levels of the dmpz were too high in energy to accept electrons from the valence-band maximum of the tin iodide. The researchers hypothesized that squeezing the material would affect the energy levels of the dmpz and tin iodide framework differently, decreasing the gap and allowing charge transfer.
|
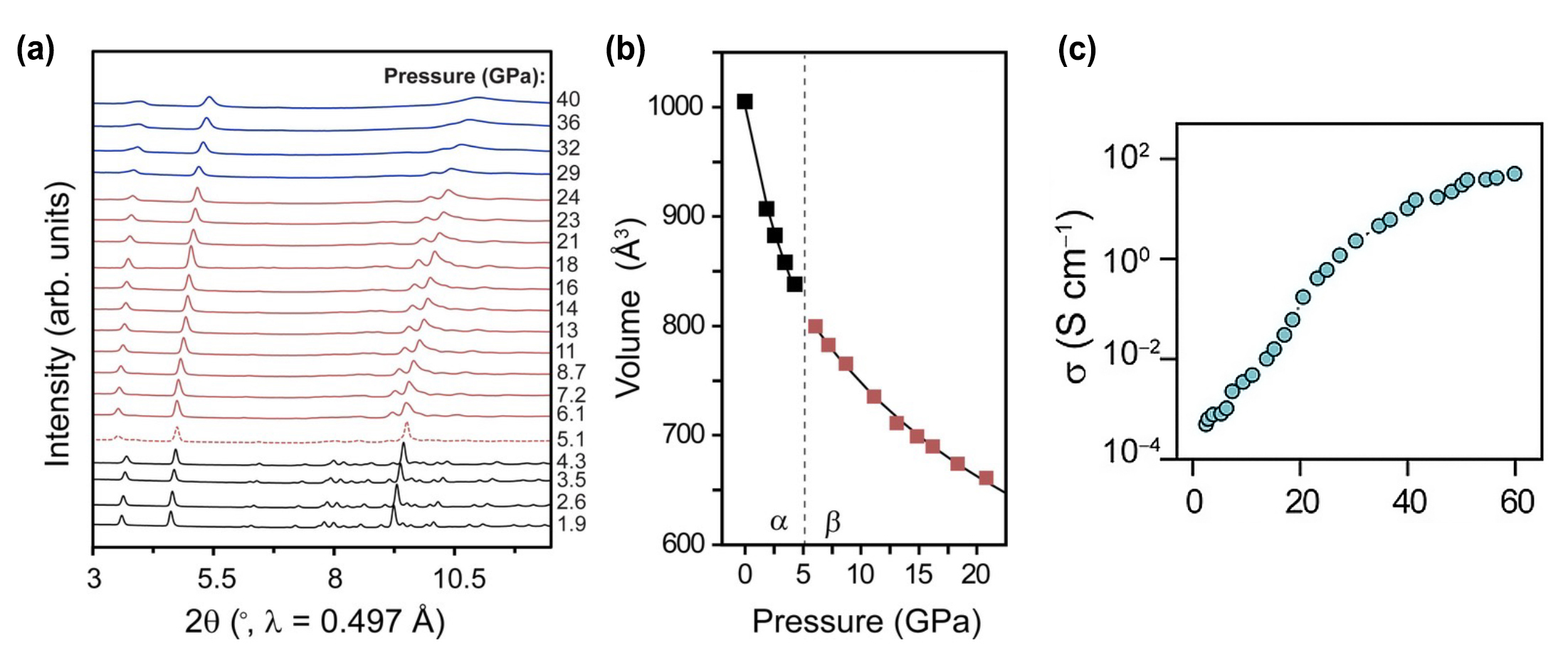 |
| (a) Powder x-ray diffraction (XRD) data for tin iodide/dmpz. Peaks shifting to the right indicates compression. Colored lines indicate different phases: α (black), α+β (red dashed), β (red solid), and γ (blue). At high pressures, the peaks broaden and then disappear, indicating that the material becomes structurally amorphous (a similar amorphization has been reported for most halide perovskites upon compression). (b)Unit-cell volume changes upon compression for α and β phases. (c) Electronic conductivity (σ) as a function of pressure. (Image: ALS)
|
Please squeeze the charges
|
|
The material’s pressure-induced structural changes were tracked using powder x-ray diffraction (up to 60 GPa) at ALS Beamline 12.2.2. Optical and transport properties were also characterized at both ambient and high pressures. The ALS measurements revealed that the material is highly compressible, nicely explaining its response to increasing pressure: the band gap nearly closed, and conductivity increased by five orders of magnitude—considerably exceeding the values achieved by related perovskites at similar pressures.
|
|
The introduction of redox-active groups to organic molecules in halide perovskites could provide delicate control over doping, pointing the way to reducing the required pressures to technologically relevant values. On a broader level, the work elevates the role of the organic molecules from templating the perovskite structure to serving as charge reservoirs for tuning conductivity, analogous to the role that charge reservoirs play in enabling high-temperature superconductivity, where decades of high-pressure investigations have afforded valuable insight into electronic properties.
|


