| May 12, 2023 |
Soaking it up: Nanoparticle sponge boosts photocatalysis
(Nanowerk News) Exciton Science researchers have created a new type of highly absorbent material that could help to increase the efficiency of photocatalysis for hydrogen production and water purification.
|
|
Based at RMIT University, the team combined silver nanoparticles with molecular organic cages to develop a new material that acts like a sponge, soaking up both sunlight and chemical reactants in a favourable manner.
|
|
When tested, this combination led to a two-fold increase in the efficiency of water splitting using the power of the sun, which is a necessary step in the creation of green hydrogen.
|
|
The results, achieved in collaboration with the University of Melbourne and Deakin University, have been published in the journal Angewandte Chemie ("Photoinitiated Energy Transfer in Porous-Cage-Stabilised Silver Nanoparticles").
|
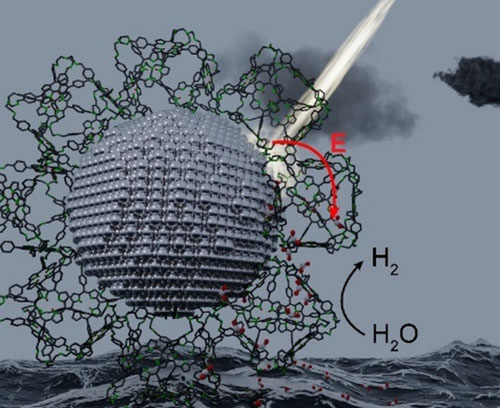 |
| A new material containing plasmonic silver nanoparticles was synthesized using only porphyrin-based porous organic cages as the ligand, which decorate the surface of the nanoparticle. The nanoparticle acts as a photosensitizer for the porphyrin cages by transferring its excited-state energy to the porphyrin. The photosensitization effect and porosity of the ligand enables superior photoelectrochemical water splitting compared to the individual components. (© Wiley-VCH Verlag)
|
|
First author Michael Wilms of RMIT said: “By increasing the surface area of highly reactive nanoparticles, we greatly enhance the conversion of water into hydrogen using only light.”
|
What is photocatalysis?
|
|
Photocatalysis promises to utilise sunlight, a near limitless source of energy, in useful chemical transformations, including water purification and the sustainable production of industrial chemical feedstocks and fuels, such as hydrogen and methane.
|
|
Efficient light-harvesting for these applications requires the development of high-performance materials capable of efficiently absorbing and converting light energy into chemicals.
|
|
Metal nanoparticles are excellent absorbers of sunlight, but are not suitable on their own for the conversion of water and CO2 into chemical fuels.
|
|
This is because ligands (the molecules which help stabilize these particles) also inhibit their ability to perform chemical reactions at their surface, the most reactive part of a nanoparticle.
|
|
Associate Professor Daniel Gomez of RMIT, the senior author on the paper and an Exciton Science Associate Investigator, said: “In this work we use completely porous ‘molecular cages’ to synthesis and stabilise the highly light-absorbing nanoparticles.”
|
|
The molecule cages are themselves highly light absorbing and can accept light energy absorbed by the metal nanoparticle, making them a ‘sponge’ for light.
|
|
Their porosity allows reactants such as water to easily diffuse inside the molecules and the metal nanoparticle, significantly increasing the efficiency of water to hydrogen conversion.
|
|
“The next step is to utilise semiconductor materials instead of metal nanoparticles which can enhance the efficiency of water splitting and allow us to trial these new materials in the conversion of CO2 as well,” Michael said.
|
|
“We are also actively exploring the replacement of silver with low-cost semiconducting materials such as zinc oxide and perovskites.”
|

