| Oct 17, 2023 |
Revolutionizing fast-charging batteries with graphite and red phosphorus nanolayers
(Nanowerk News) In a major stride towards achieving fast-charging lithium-ion batteries (LIBs) with reliable cyclability, researchers at UNIST have made a groundbreaking discovery. Their study, published in ACS Energy Letters ("Mitigating Electrode-Level Heterogeneity Using Phosphorus Nanolayers on Graphite for Fast-Charging Batteries"), introduces a novel strategy of utilizing phosphorus nanolayers to enhance the lithiation kinetics and performance of graphite-based composites, without compromising safety.
|
|
Led by Professor Hyun-Wook Lee from the School of Energy and Chemical Engineering at UNIST, the research team developed a revolutionary graphite-phosphorus composite using a vaporization-condensation method. This composite consists of a porous graphite cathode material coated with red phosphorus and carbon, enabling improved electron and lithium ion conductivity on the graphite surface.
|
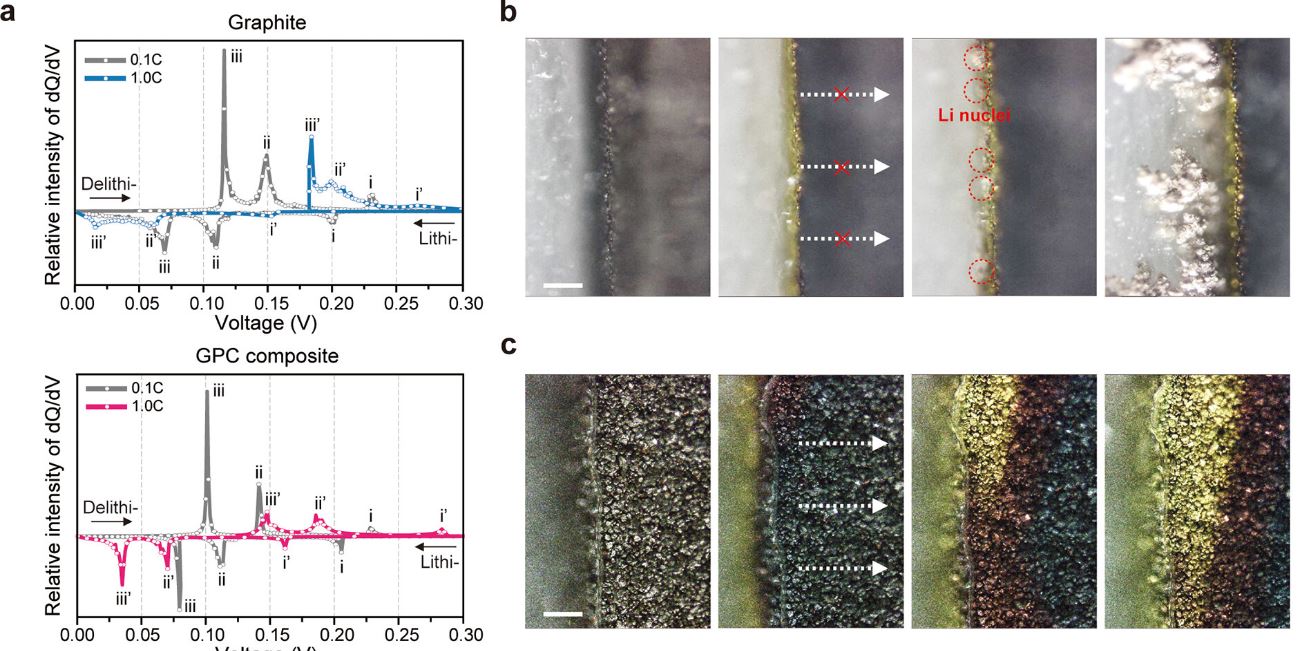 |
| Figure 1. (a) dQ dV–1 plots of bare graphite (top) and GPC composite (bottom) at C/10 and 1C. During charging at 1C, the lithiation peak (iii′) corresponding to the transformation of the LiC6 phase moves closer to 0 V in bare graphite, indicating a favorable condition for Li plating, in stark contrast to the observation for the GPC composite. (b,c) Side-view operando optical images of bare graphite (b) and GPC composite (c) at 1C. Unwanted Li plating is observed on the surface of the bare graphite but not on the GPC electrode. (Image: UNIST)
|
|
Achieving fast-charging LIBs with reliable cyclability remains a significant challenge, such as nonuniform lithiation, which can lead to performance degradation. However, the newly developed graphite-phosphorus composite electrodes exhibited a well-dispersed LiC6 phase volume fraction throughout the electrodes, indicating a homogenous lithiation process.
|
|
One of the remarkable achievements of this breakthrough is the consistent cycle retention of 94.4% and high Coulombic efficiency exceeding 99.8% over 1000 cycles at 1C. These exceptional results are attributed to the enhanced reaction kinetics of the graphite-phosphorus composite electrodes, despite their relatively high capacity.
|
|
“Graphite-based cathode materials are expected to dominate the lithium-ion battery market for the next decade,” explained Professor Lee. “Our research focuses on improving the energy density and fast-charging capabilities of graphite, which are key factors in the market.” He added, “The development of low-cost graphite cathode materials coated with red phosphorus and carbon will significantly meet the demands of the battery industry.”
|
|
The utilization of red phosphorus, with its low boiling point, allows for uniform vapor deposition on the graphite surface. By carefully controlling the deposition process, the research team successfully achieved a graphite-phosphorus composite with controlled side reactions, leading to enhanced uniformity and stability during fast charging.
|
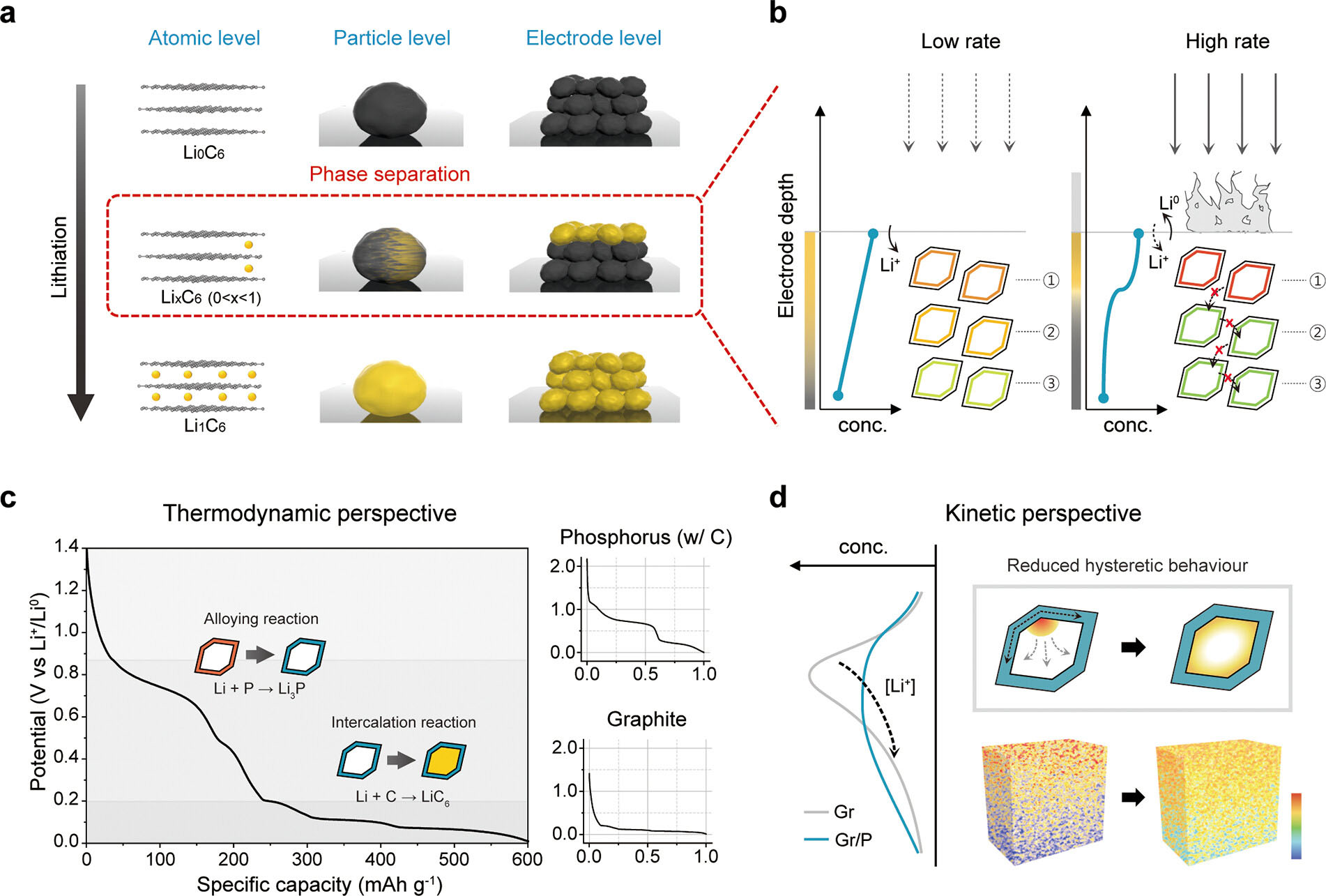 |
| Figure 2. (a) Schematic representation illustrating the reaction heterogeneity on graphite at the atomic, particle, and electrode levels. (b) Expected concentration gradient of Li ions throughout graphite electrode during charging at low and high C-rate. (c) Voltage–capacity plot of our graphite/phosphorus (GP) composites (left) and normalized voltage profiles of red phosphorus/carbon composites (right, top) and bare graphite (right, bottom). The voltage profiles provide insights into the thermodynamics of the active material ensembles. (d) Expected distribution of Li ions (and reaction) across the electrode depth in terms of kinetics of bare graphite and our composites. The phosphorus nanolayer on the graphite particles mitigates heterogeneous reactions by improving Li-ion permeation toward the bulk of the electrode. (Image: UNIST)
|
|
Real-time optical microscopy and image processing techniques were employed to observe and analyze the developed composite. The results confirmed the uniform color distribution of the graphite-phosphorus composite during charging, indicating improved lithiation uniformity. The composite also demonstrated exceptional stability, with no resin phase formation observed during fast charging, even after 1,000 cycles or more.
|
|
This breakthrough research opens up new possibilities for a wide range of applications, including electric vehicles, aviation, and fast-charging batteries. The cost-effectiveness and superior performance of the graphite-phosphorus composite make it a promising solution to overcome the challenges associated with fast-charging LIBs.
|


