| Nov 28, 2023 |
Nanodiamonds block tumor metastasis in mice
(Nanowerk News) Finding ways to contain cancer growth and prevent its spread from original sites to other body areas has posed an enduring challenge for medical researchers. Metastasis enables cancers to become more aggressive and is linked to over 90% of cancer deaths. Therapies capable of disrupting this process could thus provide lifesaving clinical advances. However, previous efforts targeting metastasis have encountered hurdles ranging from ineffective delivery to poor efficacy across cancer types.
|
|
Recent innovations in nanomedicine offer fresh opportunities to overcome historical obstacles through the engineering of customized nanoparticles. By carefully tailoring nanoparticle size, surface chemistry and more, researchers can potentially optimize delivery, binding interactions and therapeutic effects against metastasis in the body. Nanoparticles’ small size aids tissue and cell penetration, while their customizable surface chemistry permits binding with specific targets.
|
|
Carboxyl groups, for example, impart negative charge and stability in water to nanodiamonds (NDs), allowing effective interaction with cells. With low toxicity and high biocompatibility demonstrated previously, carboxylated NDs appear well-suited for biomedical applications.
|
|
A research team led by Rajiv K. Saxena from the South Asian University has taken an innovative approach, using tailored nanoparticles called carboxyl nanodiamonds (cNDs) to block metastatic tumor cell mobility and invasiveness. Their findings, reported in PNAS Nexus ("Carboxyl nanodiamonds inhibit melanoma tumor metastases by blocking cellular motility and invasiveness"), suggest cNDs represent a promising candidate for antimetastasis treatment.
|
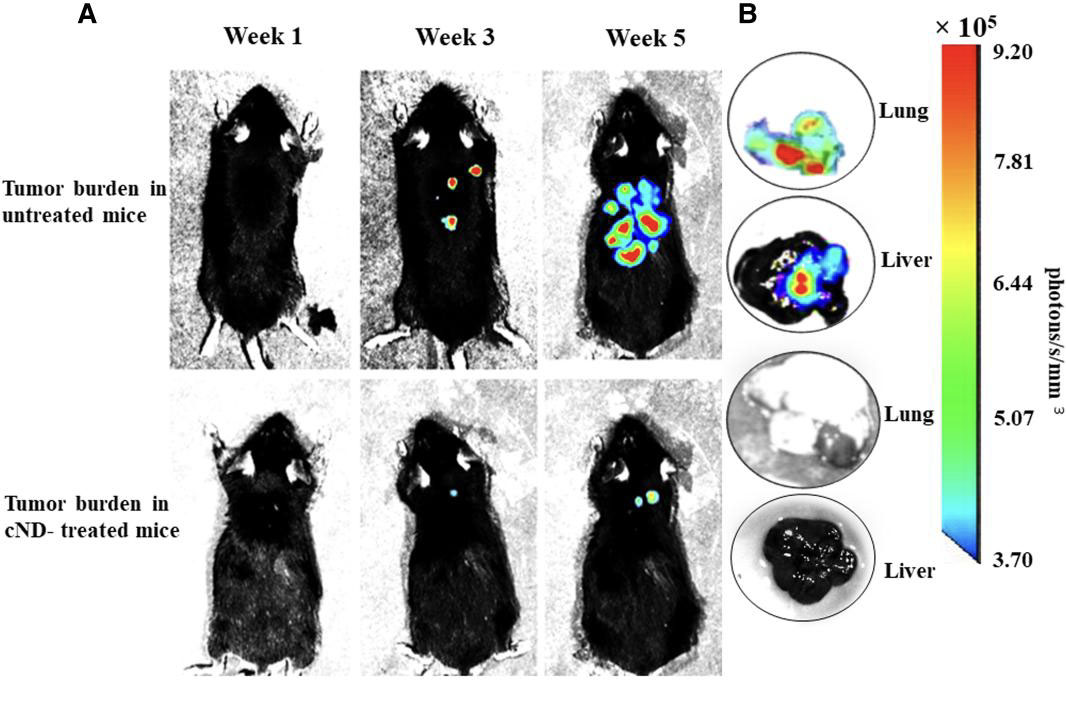 |
| Effect of treatment with cNDs on the growth and metastasis of B16F10-Luc2 tumor in mice by BLI. C57BL/6 mice were administered B16F10-Luc2 cells (5 × 105/mouse in 0.1 ml of PBS, i.v.). Each mouse in the cND-treated group of mice received 50 µl of cND suspension containing 50 μg cNDs on alternate days for 5 weeks. The mice were imaged at the end of 1, 3, and 5 weeks for the presence of the tumor cells. Ten minutes before the imaging, D-luciferin (150 mg/kg body weight of mouse, i.p.) was administered to generate the bioluminescence signals. A) Relative comparison of BLI images of tumor-bearing mice from the untreated and cNDs treated groups. B) Bioluminescence signals from lungs and livers of the untreated and cND-treated mice showing the presence/absence of tumors. Bioluminescence signal intensity scale is provided on the right of panel B. (© PNAS Nexus)
|
|
Saxena’s group set out to determine whether cNDs could disrupt the physical mechanism of cancer metastasis. Their study focused on B16F10 melanoma, a widely used preclinical metastasis model. In culture, B16F10 cells avidly took up fluorescent cNDs. The uptake of intravenously injected cNDs was verified in melanoma tumors growing within mice organs as well. This already pointed to excellent in vivo delivery potential.
|
|
Crucially, in vitro cND treatment substantially reduced B16F10 cell motility and invasiveness, key elements enabling metastasis. cND-treated cells showed difficulties traversing micropore membranes, used to mimic natural tissue barriers. Their migration and invasion defects reflected changes in relevant secreted and intracellular proteins. Secretion of matrix metalloproteinases MMP-2 and MMP-9 fell dramatically with cND treatment. These MMP enzymes normally help degrade local tissues, assisting metastasizing cancer cells’ spread.
|
|
Intracellularly, cNDs also reduced amounts of metastasis-promoting factors like β-catenin and vimentin while increasing levels of junction proteins E-cadherin and claudin-1. This protein modulation likely contributes to the observed mobility suppression.
|
|
Translating these movement and invasiveness impairments to actual metastasis blockade in animals would prove even more significant. So Saxena’s team examined intravenously injected, luciferase gene-modified B16F10 effects in mice, with or without alternate-day cND treatment. Bioluminescence imaging dynamically tracked tumor growth, revealing striking differences between groups.
|
|
While control mice suffered rapid tumor expansion into thoracic and abdominal regions, cND treated mice showed hardly detectable tumor signals even after 5 weeks. Examining dissected organs confirmed drastically fewer melanoma infiltrations with cND therapy.
|
|
Survival periods also diverged markedly. Observing comparable primary subcutaneous tumor growth with and without cNDs further isolated their antimetastasis action. Visible inspection and histology verified near total absence of organ metastases with cND treatment in this model as well. No noticeable toxicity or adverse effects arose for the mice from long-term cND administration according to weight, appearance and tissue analysis too.
|
|
Through mechanistic examination and therapeutic testing, these findings compellingly demonstrate cNDs’ potent metastasis suppression in B16F10 melanoma, with wide-ranging implications. Saxena explains cND interference using a well-established seven step model of metastasis physiology. Early tumor survival and vascularization processes seem unaffected.
|
|
Several later stages though rely strongly on cancer cell motility, invasiveness and extracellular matrix degradation. By coordinately inhibiting cellular traits underlying each of those steps, cNDs disrupt the complete metastases cascade.
|
|
This generalized blockade across metastatic route stages and cell types heightens applicability beyond the present work. Inflammation also critically assists metastasis, offering another target for cNDs’ proven anti-inflammatory properties.
|
|
With such a multifaceted chemical-biological antimetastasis mechanism identified, these tailored nanodiamonds could significantly impact clinical outcomes. Expanding evaluation across diverse metastasis models can further optimize treatment protocols before human trials.
|
|
Combination with localized primary tumor therapies may improve survival chances as well. But the urgent need for progress against lethal metastasis already positions cNDs as an exciting near-term prospect in this vexing fight against cancer. Their translational development could spark major advances in years ahead.
|

