| Mar 13, 2024 |
Chemists develop machine learning model for atomic-level interactions
(Nanowerk News) What exactly happens at the tiny scale at which individual atoms exist and interact? SMU chemist Elfi Kraka and her colleagues have been working on developing a computational tool aimed at providing answers to that mystery.
|
|
Mathematical functions used to calculate the potential energy of a system of atoms are called interatomic potentials. Machine learning interatomic potentials (MLIP)s have become an efficient and less expensive alternative to traditional quantum chemical simulations, which even on today’s high-performance computing often become out of reach for larger systems. In their quest, the researchers developed a novel MLIP, called ANI-1xnr that is applicable to a broad range of reactive chemistry without the need for costly refitting, a major drawback of the MLIPs currently in use.
|
|
Their findings appear in the journal Nature Chemistry ("Exploring the frontiers of condensed-phase chemistry with a general reactive machine learning potential").
|
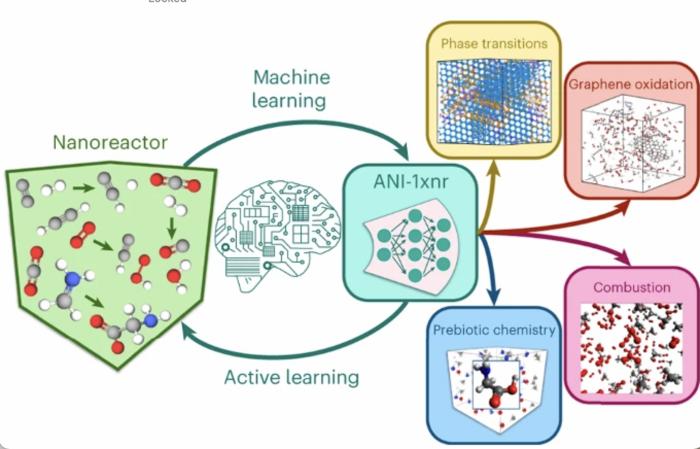 |
| Researchers developed a general reactive MLIP (ANI-1xnr) through automated sampling of condensed-phase reactions. ANI-1xnr was then applied to study five distinct systems: carbon solid-phase nucleation, graphene ring formation from acetylene, biofuel additives, combustion of methane and the spontaneous formation of glycine from early earth small molecules. (© Nature Chemistry)
|
|
“Understanding interactions at the atomic level could aid us in caring for our planet, impact the ways drugs interact with the human body, and even let us explore organic materials that have come to Earth thanks to comets and asteroids or simulate chemical reactions taking place far out in space including star dusts or interstellar grains,” Kraka said.
|
|
Kraka and her colleagues put their new ANI-1xnr potential to the test, simulating five distinct systems in extreme environments: carbon solid-phase nucleation, graphene ring formation from acetylene, biofuel additives, combustion of methane and the spontaneous formation of glycine from early earth small molecules (the famous Miller experiment), with all five closely matching available experimental or theoretical data.
|
|
Miller conducted an experiment in 1959, applying electricity to a mixture of simple molecules, such as ammonia, carbon monoxide, water, hydrogen, and methane uncovering how amino acids, important life-building blocks, were formed. His experiment created a new field of study called prebiotic chemistry, which aims to understand the chemical processes and reactions that occurred on Earth before the emergence of life. Since then, scientists have referred to Miller's experiment to understand specific reactions that lead to the formation of amino acids under Early Earth conditions.
|
|
The ANI-1xnr dataset is now available publicly for research use. The authors intend expand and further train ANI-1xnr over time, where Kraka is particularly interested in exploring the formation of amino acids and precursors of DNA from small molecules under extraterrestrial conditions, one of her recent research interests.
|

