| Posted: Jun 21, 2017 | |
Gold nanoclusters possess surprisingly high wide-spectrum antimicrobial activity(Nanowerk News) Researchers in Singapore have demonstrated that it is possible to confer antimicrobial activity to gold nanoparticles, which have long been considered as inert, by way of controlling their sizes to the nanocluster range. |
|
| As the team reports in ACS Nano ("Antimicrobial Gold Nanoclusters"), they found that sub-2 nm sized gold nanoclusters showed antimicrobial effect that was absent from their larger counterpart, gold nanoparticles (>2 nm). | |
| The researchers found that gold nanoclusters could kill both Gram-positive and Gram-negative bacteria, showing their potential as a wide-spectrum bactericide. | |
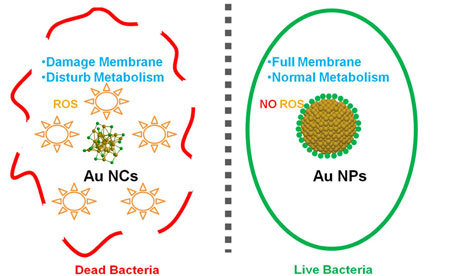
| Unlike silver, gold is inert, highly stable, and would not easily dissociate into ions. These qualities contribute to the widely accepted notion of gold nanoparticles as being highly biocompatible in mammalian system, both in vitro and in vivo. This biocompatibility in mammalian cells is also observed when the size is further reduced to the nanocluster range. | |
 |
|
| Nanoparticles' size: The defining line between life and death. (© ACS) | |
| Gold's inertness led many in the field assume that gold would not be a viable antimicrobial material. Instead, gold nanoparticle-based antimicrobial strategies evolved around grafting known antimicrobial compounds, such as ampicillin, antimicrobial peptides, and cationic or zwitterionic ligands, on the surface of large gold nanoparticles, presumably using gold nanoparticles as passive drug carriers. | |
| Now, since an enhanced antimicrobial activity was observed when the size of the gold nanoparticles was reduced to the nanocluster range, the authors hypothesized that through size control, gold nanoparticles, when reduced down to gold nanoclusters, might begin to exhibit some special biological properties, such as antimicrobial activity, contrary to the popular assumption of inertness. | |
| In their paper, the scientists demonstrated that ultrasmall gold nanoclusters possess surprisingly high wide-spectrum antimicrobial activity, which is however absent in their larger gold nanoparticles counterparts. | |
| In addition, they elucidated the possible mechanism that drives the antimicrobial activity of ultrasmall gold nanoclusters. This wide-spectrum antimicrobial activity is attributed to the gold nanoclusters’ ultrasmall size, allowing them to possess high surface to volume ratio. | |
| Consequently, when the gold nanoclusters were internalized, this active surface could induce a metabolic imbalance in the cells, which leads to an increase of intracellular reactive oxygen species production, conferring bacteria killing effect as the end result. |
| Source: American Chemical Society | |
|
Subscribe to a free copy of one of our daily Nanowerk Newsletter Email Digests with a compilation of all of the day's news. |
