| Posted: November 17, 2009 |
Single molecule spectroscopy shows how genetic material is unwrapped |
|
(Nanowerk News) The genetic material found in cells is not in its free state, but is bound to large protein complexes and tightly wrapped. To activate genes that could well play a role in carcinogenesis, the genetic material first needs to be unwrapped and made accessible to other cell components. Using a new biophysical method called single molecule spectroscopy, scientists from the “Biophysics of Macromolecules“ division at the German Cancer Research Centre (Deutsches Krebsforschungszentrum, DKFZ) were the first to directly observe these mechanisms and characterise the intermediate stages leading to free genetic material. Their results have recently been published in the Proceedings of the National Academy of Sciences ("Nucleosome disassembly intermediates characterized by single-molecule FRET").
|
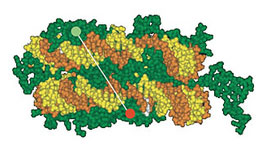 |
| The DNA double strand (yellow and orange) is wrapped around a histone complex (green) consisting of eight subunits.
|
|
Every cell in the body contains the genetic material DNA in its nucleus. Under normal conditions, DNA is not found in its unbound state, i.e., as a long, thread-like molecule, but is packaged into a compact structure. The DNA is wrapped around protein complexes called histones to form a structure that resembles a string of beads. Scientists refer to the smallest packaging unit of DNA - one bead on the string - as a nucleosome; this is made up of eight histones and the DNA bound to them.
|
|
The DNA double strand is wrapped around a histone complex consisting of eight subunits. To ensure that basic processes such as DNA replication during cell division or the translation of genetic information into proteins is able to take place without problems, enzymes need to have free access to genetic information. Therefore, it is vital that the genetic material can be converted back from its compact form into a form that is easier to access. To make this happen, the cell regulates the binding of DNA to the histones through chemical changes in the histones themselves. In this way, these changes influence both the structure of the genetic material and the cell function. Since activation and silencing of genes or oncogenes also depend on the extent of packaging, these chemical changes are believed to play a central role in the transformation of a normal cell into a cancer cell.
|
|
When a gene is activated, the DNA is unwrapped from the histones and the protein complexes can disassemble into smaller subunits; the nucleosome then opens up. During the reverse process, the nucleosome closes again, the histone proteins reassemble and the genetic material is wrapped around the histone protein complex.
|
|
It only recently became possible to study such processes directly in single proteins and DNA molecules. Single molecule spectroscopy is a modern analysis tool which uses a laser beam with a focus of one thousandth of a millimetre to ‘count’ and characterise molecules one by one. This method makes it possible to analyse different states of biomolecules both in isolated form in the test tube and in living cells. Thus, scientists are able to directly observe individual steps of biological processes.
|
|
Using single molecule spectroscopy, scientists in Professor Jörg Langowski’s team at the Division of Biophysics of Macromolecules at the DKFZ studied the mechanism that leads to the unpacking of DNA and, thus, the activation of a gene. They discovered that the genetic material is released from the protein complex in a stepwise process where the protein complexes disassemble gradually. The researchers were able to characterise several intermediates during the disassembly of the histone proteins and were thus able to describe these processes in detail. They succeeded in directly detecting the intermediates that form during the opening of the nucleosome. These findings are important for understanding the mechanisms by which genes are switched on and off through changes in the histones and might help to answer the question relating to how oncogenes are activated. The findings also offer a target for developing anti-cancer drugs, several of which are already being tested in clinical trials.
|

