| Posted: December 25, 2009 |
Method to detect cancer cells utilizing the bioluminescence reaction of Cypridina noctiluca |
|
(Nanowerk News) Researchers in Japan have developed a technique to locate cancer cells by synthesizing a near-infrared light-emitting protein and then conjugating it with a therapeutic antibody to produce a probe for cancer cells.
|
|
The near-infrared light-emitting protein was synthesized by introducing a near-infrared light-emitting organic fluorescent dye in the sugar chain of Cypridina luciferase. This fluorescent dye emits near-infrared light when energy transfer occurs in in vivo chemical reactions. Because near-infrared light can easily penetrate living tissue, the near-infrared light emitted within the body can be observed externally. This type of near-infrared light-emitting protein has never been synthesized before.
|
|
Moreover, the researchers demonstrated that the liver cancer cells implanted within the body of a mouse could be monitored using the near-infrared light-emitting probe along with a CCD camera.
|
|
The probe was produced by conjugating the near-infrared light-emitting protein with a therapeutic antibody protein. This newly developed probe for cancer cells emits near-infrared light by chemical reactions. Thus, this method for cancer cell detection does not require external irradiation with radiation or ultraviolet light. This probe can be used for the assessment of therapeutic antibodies, real-time pathological diagnosis, etc., and can lead to innovations in medical technology.
|
|
The details of this study have been published in the Proceedings of the National Academy of Sciences of the United States of America (PNAS) on September 7, 2009.
|
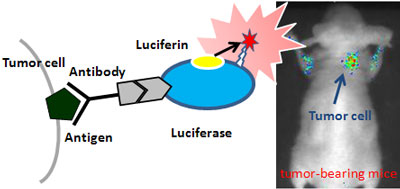 |
| Schematic diagram of near-infrared light-emitting probe
|
|
Social Background for Research
|
|
It is important to develop cancer medicine to protect the health of Japanese people, and the development of intraoperative photo-dynamic diagnosis that can enable the complete removal of minute cancer tissues without the need for large-scale facilities is highly anticipated. On the other hand, the use of monoclonal antibodies as new cancer therapeutics (the so-called therapeutic antibodies) has attracted considerable attention. Therapeutic antibodies recognize cancer-cell-specific antigens and attack cancer cells. Therefore, it is very important to elucidate the in vivo behaviors of therapeutic antibodies in order to assess the antibodies. Moreover, minute cancer tissues can be identified by visualizing the cancer cells using antibodies.
|
|
Currently, techniques such as positron emission tomography (PET) using an accelerator, are used to monitor the in vivo migration of antibodies; however, PET requires large-size equipment and large-scale facilities, is time-consuming, and is expensive, which greatly limits the use of PETs in clinical environments. Although optical probes, such as green fluorescent protein (GFP), can also be used to monitor therapeutic antibodies, the range of fluorescence of GFP is within the range of visible light (wavelength: 400–700 nm) which cannot easily penetrate living tissue; therefore, the use of GFP is limited. An alternative to GFP is utilization of near-infrared light (wavelength: 700–1400 nm), which can easily penetrate living tissue through the so-called “optical window (wavelength: 650–1000 nm)”. However, although the use of near-infrared light has been highly anticipated, it has seldom been demonstrated in practical applications.
|
|
History of Research
|
|
AIST has recognized the considerable potential of bioluminescence system of Cypridina noctiluca and has been conducting research in the large-scale production of Cypridina luciferin and Cypridina luciferase. In collaboration with Graduate School of Medicine, Hokkaido University and Liv Tech Inc., AIST has been developing a light-emitting probe, namely, a light-emitting protein conjugated with DLK-1 antibody (one of candidate therapeutic antibodies) as well as applications of the probe for the visualization of liver cancer cells.
|
|
Details of Research
|
|
The near-infrared light-emitting protein, which emits near-infrared light by the in vivo chemical reactions via artificial bioluminescence resonance energy transfer (BRET), was synthesized by introducing a near-infrared light-emitting organic fluorescent dye to the sugar chain of Cypridina luciferase. Because near-infrared light is barely absorbed by the hemoglobin in blood (Fig. 1), near-infrared light emitted within the living body can be monitored externally using, for example, a CCD camera. Previously developed near-infrared light-emitting fluorescent dyes require external light sources to supply the energy required for the emission of fluorescent light. However, the newly developed near-infrared light-emitting protein does not require an external light source because it can emit light by in vivo chemical reactions.
|
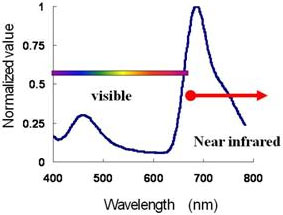 |
| Figure 1. Emission spectrum of the near-infrared light-emitting protein and Cypridina luciferin in blood
|
|
The near-infrared light-emitting probe was produced by conjugating a near-infrared light-emitting protein with DLK-1 antibody, one of therapeutic antibody candidates. This probe was added to the cell culture medium containing DLK-1 antigen-expressing cells, and incubated for approximately 120 minutes. After the cells were washed, Cypridina luciferin was added to the medium. As a result, emission of near-infrared light was observed only in the DLK-1 antigen-expressing cells. This indicates that the near-infrared light-emitting probe has two abilities: to recognize the DLK-1 antigen and to emit near-infrared light.
|
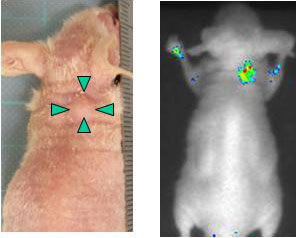 |
| Figure 2: Appearance of the mouse with implanted liver cancer cells (left) and the real-time image of cancer cells expressing DLK-1 obtained using the near-infrared light-emitting probe
|
|
Cancer cells expressing the DLK-1 antigen were implanted in mice. When the implanted cancer cells grew to approximately a few mm, the near-infrared light-emitting probe was intravenously injected into the mice. Twenty-four hours after the probe injection, Cypridina luciferin was intravenously injected into the mice. Emission of near-infrared light was observed at the site of cancer cell implantation when the mice were imaged using a CCD camera (Fig. 2). A filter that blocks light of wavelength less than 570 nm was used to observe the emission of light in the wavelength range around the near-infrared region, and clear images with little background were obtained.
|
|
Future Schedule
|
|
We will apply this newly developed technique to various antibodies, and develop many applications including the development of antibody therapeutics, antibody-based intraoperative diagnosis, real-time pathological imaging, and antibody-based assessment of regenerative cells.
|



